INTRODUCTION
The pulmonary and bronchial arteries represent the two main sources of lungs’ blood supply. Although the bronchial arteries (BAs) account for only 1% of the cardiac output, they are able to provide additional systemic circulation to the lungs in several thoracic disorders. In order to identify possible BA anomalies and dilatation, it is essential to understand the BA anatomy and function. The BAs most commonly originate from the descending aorta at the level of the upper thoracic vertebral bodies (T5–T6)1. The most common finding is the presence of two left and one right main BA (approximately 70% of patients).
The BA dilates in several pathological conditions, involving BA anomalies, acute or chronic inflammation of the lungs and airways, and pulmonary hypertension2,3. In such cases, increased arterial blood flow via the BA and anastomotic vessels may result in arteriolar rupture, leading to variable degrees of hemoptysis4. Actually, the high-pressure BA circulation is the cause of hemoptysis in the majority of patients (90%)5. Rarely, the aorta and the bronchial and pulmonary veins may be the source of hemoptysis.
Massive hemoptysis, or life-threatening hemoptysis, does not have a universally established definition yet, but bleeding rate of more than 100 mL/h, or total blood volume of more than 500 mL in 24 hours, are considered life-threatening6. Diagnostic evaluation of massive hemoptysis includes the bleeding site localization, and the detection of the underlying cause, which can be promptly performed with chest computed tomography (CT) scan in patients who present with sufficient oxygenation, and are hemodynamically stable7. Bronchoscopy is considered valuable for some patients, particularly when a CT scan can indicate the bleeding site8. However, the more widespread availability of bronchial artery embolization (BAE), which has been characterized by a high rate of complete cessation of hemoptysis, has led to a crucial management modification of massive hemoptysis9,10. BAE has become the primary intervention to control bleeding in the acute setting, instead of surgical resection5. Recently, CIRSE published a document recommending a rational approach to hemoptysis and best methods for performing BAE11.
We present a patient who attended the emergency department with massive hemoptysis, and was treated with superselective embolization due to an anatomical variation. Clinically, a missing anatomical variation can result in treatment failure and reduce hemoptysis-free survival rate12. Therefore, it is of great importance to recognize the anatomy and the variants of BA.
CASE PRESENTATION
A 44-years-old man, current smoker with a smoking history of 50 packyears, presented at the emergency department because of recurrent episodes of small volume hemoptysis. His medical history revealed dyshidrotic eczema and hiatus hernia. His regular medication included acetylsalicylic acid (100 mg/day).
Upper airway evaluation did not reveal any sign of local hemorrhage. The chest CT scan revealed ground glass opacities in the right middle lobe and mild bronchiectasis. CT pulmonary angiogram (CTPA) was negative for pulmonary embolism, while inflammatory markers, QuantiFERON test and serology tests including immunological testing (complement C3 and C4, immunoglobulins, as well as antineutrophil cytoplasmic antibodies, antinuclear antibodies, anti-glomerular basement membrane antibodies, rheumatoid factor, anticyclic citrullinated protein anti-bodies, antidouble stranded DNA antibodies, and anti-extractable nuclear antigen antibodies), were all within normal limits. Bronchoscopy at that time revealed normal findings. Hemoptysis gradually slowed and stopped within 48 hours, whilst the patient received only conservative treatment and discontinued acetylsalicylic acid.
During the next month the patient was hospitalized three times, while each time the vital signs, the hemoglobin levels and the SatO2 levels (97% on room air) were not affected.
However, ten days after the third hospital discharge, he presented with hemoptysis of 150 mL in 15 minutes and SatO2 of 91%. Emergent intubation was required for airway control in this case of massive hemoptysis; therefore, the patient was admitted to the intensive care unit (ICU). New CT scan revealed extension of ground glass opacities (Figure 1A). Bronchoscopy was performed in the ICU, which detected a blood clot in the bronchus intermedius.
Figure 1
A) Lung window form CT pulmonary angiogram images on presentation showed extensive ground glass opacities in the right middle and lower lobe; B, C) 10 days after BAE, CT images revealed the extinction of ground glass opacities. Mild bronchiectasis could be also detected
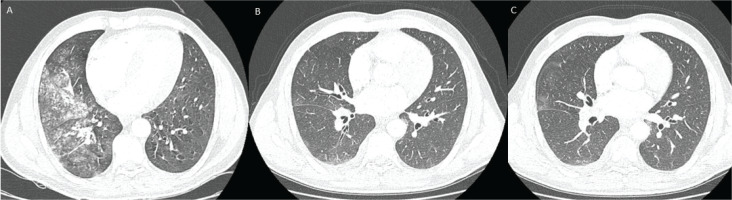
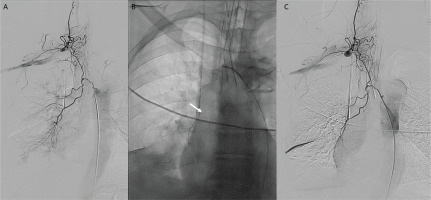
The patient was then transferred to the interventional radiology (IR) department, where common femoral artery was punctured, and descending thoracic aortogram was performed without significant findings. Subsequently, right intercostobronchial trunk was catheterized using an angled vertebral catheter and selective angiography was performed. Digital subtraction angiography (DSA) showed no significant tortuosity or hypertrophy of BA. Nevertheless, an extensive communication with right thyrocervical trunk was revealed, suggesting a double origin of the artery (Figure 2A). To prevent non-target-site embolization and significant possible complications, superselective catheterization of its branches was performed. A 2.4 F microcatheter (Merit Maestro, Merit Medical) (Figure 2B) and a steerable 0.018 inch guidewire (Tenor, Merit Medical) was used, followed by transarterial embolization with microspheres 300–500 μm (Embosphere, Merit Medical). Careful pulsatile injection of embolic material, under active fluoroscopy was applied to confirm any signs of reflux. Final DSA confirmed flow stasis in target branches (Figure 2C). After the procedure, there was significant clinical improvement without new episodes of hemoptysis. A new CT scan was performed 10 days later, which detected elimination of ground glass opacities (Figures 1B and 1C).
Figure 2
A) Selective DSA from right intercostobronchial trunk revealed an extensive communication with right thyrocervical trunk; B) Superselective catheterization of bronchial artery branches was performed with a microcatheter (arrow) followed by transcatheter embolization with microspheres 300–500 μm; C) Final DSA showed flow stasis in target branches
CONCLUSION
The patient described in this report presented with massive hemoptysis and underwent thorough investigation to rule out other diagnosis, including infection, pulmonary embolism, parenchymal disorders, and vasculitis. BAE was necessary to treat massive hemoptysis. However, diagnostic DSA revealed that it was an extremely rare case of double origin of right bronchial artery, that required superselective catheterization to prevent serious embolization complications.
BAs mostly originate from the upper descending thoracic aorta. However, there have been described several anatomical variances (origin, branching patterns)13,14. A recent study included 600 patients with hemoptysis and determined the orthotopic and ectopic BA anatomy, using multidetector computed tomography (MDCT) and digital subtraction angiography (DSA)15.
Ectopic origin can be found in 30% of patients and includes origin from aorta outside T5-T6 level or other arteries such as internal thoracic artery, subclavian artery, thyrocervical trunk, or coronary arteries16. However, the presence of double origin is very rare2. CT has emerged as an important non-invasive tool in the evaluation of patients with hemoptysis. An appropriate CT angiography protocol is necessary to ensure not only opacification of the bronchial arteries, but also opacification of pulmonary and other systemic arteries, that may contribute to hemoptysis17.
The alternative anatomy of the aortic arch is of great clinical importance, not only from a surgical point of view18, but also during embolization and catheterization in the IR department. BAE is a generally safe procedure, although it can be related with serious complications, including transverse myelitis, stroke, transient cortical blindness, ischemic colitis, and dysphagia, as a result of non-target embolization11. It is worth highlighting that not only diagnostic and interventional radiologists, but also pulmonologists and intensivists should be aware of the spectrum and variations of the bronchial artery system.
Conclusively, careful review of the initial digital subtraction angiography is essential to detect anatomical variations. Prevention of non-target embolization and avoidance of serious complications can be achieved using superselective embolization.



