INTRODUCTION
COVID-19 is the disease caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which was first detected in Wuhan, China in December 2019. COVID-19 has dominated clinical practice during two pandemic waves in our country as well as globally. New aspects of the clinical presentation of COVID-19 infection continue to emerge as more patients are infected and require hospitalization. Here, we present the case of a patient with COVID-19 infection who developed spontaneous pneumomediastinum and subcutaneous emphysema.
CASE PRESENTATION
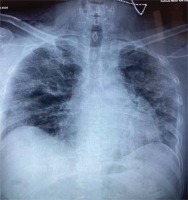
A 62-year-old non-smoker male patient, with no medical history, was admitted to the 1st COVID department of our hospital on day 9 of COVID-19 infection, due to progressive shortness of breath. The disease had been confirmed by SARS-CoV-2 reverse transcription-polymerase chain reaction assay (RT-PCR) 7 days prior to his admission and he had received azithromycin for 5 days and methylprednisolone per os for the past 2 days. On admission, he presented with bilateral, widespread fine crackles on auscultation and respiratory failure (pO2/FiO2 ratio: 285), WBC of 12.000 K/ μL, CRP of 6 mg/dL (normal values <0.5 mg/dL), D-Dimers 0.6 μg/mL (normal values <0.5 μg/mL) and ferritin of 500 ng/mL. His chest X-ray is shown in Figure 1.
Treatment with remdesivir (100 mg od after an initial dose of 200 mg iv) and recombinant human interleukin-1 receptor antagonist (100 mg od) was initiated, while methylprednisolone was continued (60 mg od iv). Oxygen therapy was gradually escalated during the first days of hospitalization and by day 12 of illness the patient was on High Flow Nasal Cannula (HFNC) 60 lpm and FiO2 95% (pO2/FiO2 ratio: 69) as well as in prone position for most hours of the day.
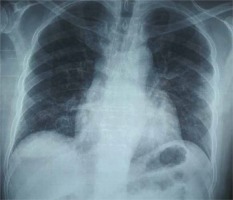
On day 15 of illness, while the patient continued to be severely ill (pO2/FiO2 ratio: 75; mostly in prone position on HFNC 60 lpm FiO2 90%, dyspneic at minimal effort without signs of hemodynamic compromise) a new chest X-ray showed pneumomediastinum and subcutaneous emphysema of the neck and chest wall (Figure 2). A subsequent chest computed tomography (CT) demonstrated the extension of the pneumomediastinum and subcutaneous emphysema and revealed diffuse bilateral ground glass opacities (Figure 3). The patient reported no pain of the chest or neck and no new onset dyspnea.
Figure 2
Routine Chest X-Ray on Day 15 of illness. Presence of air around the aorta (Ring Sign- black arrow), pneumo-mediastinum, subcutaneous emphysema of the neck and chest wall along with diffuse bilateral airspace opacities
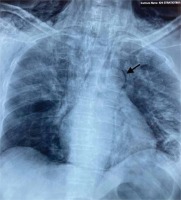
Figure 3
Chest CT on day 15 of illness. Presence of pneumo-mediastinum, subcutaneous emphysema of the thoracic wall and the neck as well as diffuse bilateral ground glass opacities. There is a trace of pneumothorax in the left lung (red arrow)
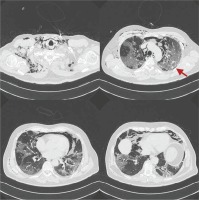
The pneumomediastinum and the subcutaneous emphysema were managed conservatively, while pharmacological treatment and oxygen therapy for COVID-19 were continued. On day 18 of the disease, a new chest X-ray showed complete resolution of the pneumomediastinum and the subcutaneous emphysema (Figure 4). The patient remained clinically stable and respiratory support was gradually deescalated until discharge.
DISCUSSION
Pneumomediastinum (PM) is defined as the presence of air in the mediastinum and is characterized as either secondary or spontaneous1,2. The latter appears as the result of either trauma or iatrogenic procedures, such as intubation or central line placement1,2. Spontaneous pneumomediastinum (SPM) refers to the cases in which such traumatic history is absent, but there is an association with preexisting respiratory disease (such as asthma, interstitial lung disease and recent respiratory infection) and certain triggering events (such as persistent cough and strenuous physical activity)2. In the case presented here, SPM was diagnosed in view of absence of traumatic or iatrogenic causes, albeit the patient had no previous history of respiratory disease and did not suffer from persistent cough.
Although a few sporadic cases of SPM in the course of other viral respiratory illness3-6 were reported, there is growing evidence of SPM linked to COVID-19, already during this first year of the pandemic7-40. Despite their relatively small number, there is marked heterogeneity regarding the diagnostic approach, the need for noninvasive (NIV) or mechanical ventilation, the degree and means of oxygen therapy delivered, the co-presence or absence of pneumothorax and subcutaneous emphysema (SE), and clinical outcomes (Table 1).
Table 1
Total of cases appearing in PubMed search regarding SPM and COVID-19. There are a total of 101 cases of SPM related to COVID-19, originated in a variety of countries. Among the 101 patients with SPM, 24 patients had concomitant pneumothorax (23.7%) and 64 (63%) were found with concomitant SE. There were 25 (24.7%) patients who had received lung protective invasive ventilation and only 2 patients on NIV, prior to SPM diagnosis. Rest of the patients (73%) were delivered oxygen therapy via oxygen masks and 1 patient was on HFNC
| Author | Country | Number of patients with SPM | SARS-Cov-2 infection confirmation | Mechanical ventilation prior to SPM diagnosis | Concomitant pneumothorax | Concomitant Subcutaneous emphysema |
|---|---|---|---|---|---|---|
| Lemmers et al.7 | Italy | 23 | RT-PCR | 23/23 (intubated in ICU) | 0/23 | 23/23 |
| Manna et al.8 | USA | 11 | RT-PCR | 0/11 | 1/11 | 11/11 |
| Diaz et al.9 | USA | 3 | RT-PCR | 0/3 | 1/3 | 0/3 |
| Mohan et al.10 | USA | 1 | RT-PCR | 0/1 | 0/1 | 1/1 |
| Gorospe et al.11 | Spain | 4 | RT-PCR | 0/4 | 0/4 | 2/4 |
| Urigo et al.12 | UK | 1 | RT-PCR | 0/1 | 0/1 | 1/1 |
| Quincho-Lopez et al.13 | Peru | 2 | (+) COVID-19 IgM, IgG | 0/2 | 1/2 | 0/2 |
| Zhou et al.14 | China | 1 | RT-PCR | 0/1 | 0/1 | 0/1 |
| Wang et al.15 | China | 1 | RT-PCR | 0/1 | 1/1 | 1/1 |
| Wang J et al.16 | China | 1 | RT-PCR | NIV 1/1 | 0/1 | 0/1 |
| Loffi et al.17 | Italy | 6 | RT-PCR | 0/6 | 0/6 | 3/6 |
| Shan et al.18 | China | 1 | RT-PCR | 0/1 | 1/1 | 1/1 |
| Lopez Vega19 | Spain | 3 | RT-PCR | 0/3 | 2/3 | 0/3 |
| Al-Azzawi et al.20 | USA | 3 | RT-PCR | 2/3 | 0/3 | 3/3 |
| Eperjesiova et al.21 | USA | 7 | n/a | 0/7 | 2/7 | 4/7 |
| Marsico et al.22 | Spain | 1 | n/a | 0/1 | 0/1 | 1/1 |
| Jansssen et al.23 | The Netherlands | 1 | RT-PCR | 0/1 | 0/1 | 0/1 |
| Elhakim et al.24 | USA | 1 | RT-PCR | 0/1 | 1/1 | 1/1 |
| Mimouni et al.25 | Morocco | 1 | RT-PCR | 0/1 | 0/1 | 0/1 |
| Kong at al.26 | China | 1 | RT-PCR | 0/1 | 0/1 | 0/1 |
| Hazariwala et al.27 | USA | 2 | RT-PCR | 0/2 | 2/2 | 2/2 |
| Gillepsie et al.28 | USA | 1 | RT-PCR | 0/1 | 1/1 | 1/1 |
| Colmenero et al.29 | Spain | 1 | n/a | 0/1 | 1/1 | 0/1 |
| Kolani et al.30 | Morocco | 1 | RT-PCR | 0/1 | 0/1 | 0/1 |
| Belini et al.31 | Italy | 1 | RT-PCR | 0/1 | 0/1 | 0/1 |
| Juάrez-Lloclla et al.32 | Spain | 11 | n/a | 0/11 | 5/11 | 5/11 |
| Brogna et al.33 | Italy | 2 | RT-PCR | 0/2 | 1/2 | 0/2 |
| Li et al.34 | USA | 1 | RT-PCR | 0/1 | 0/1 | 0/1 |
| Oye et al.35 | USA | 2 | RT-PCR | 0/2 | 2/2 | 0/2 |
| Pingui et al.36 | China | 1 | RT-PCR | 0/1 | 0/1 | 0/1 |
| Tucker et al.37 | USA | 2 | RT-PCR | 0/2 | 1/2 | 1/2 |
| Zayet et al.38 | France | 1 | RT-PCR | 0/1 | 1/1 | 1/1 |
| Vazzava et al.39 | Italy | 1 | RT-PCR | NIV 1/1 | 0/1 | 1/1 |
| Wegner et al.40 | UK | 1 | RT-PCR | 0/1 | 0/1 | 1/1 |
In our case, the patient had no clinical or radiological signs of SE or PM on admission and we believe that he developed SPM between day 10 and day 14 of disease, while being hospitalized (Figure 1). This appears in line with similar cases reported in non-intubated patients where the mean time of SE/SPM appearance was 13.3 days12.
PM most commonly presents with chest pain; however, up to 50% of SPM cases are characterized by the absence of specific clinical symptoms1. Accompanying SE can be detected in up to two-thirds of patients with PM cases and is usually characterized by neck pain and swelling1. In our case, there were no symptoms or signs of clinical deterioration when SPM and SE occurred, with chest radiography revealing these complications and providing a more accurate understanding of the patient’s clinical status. This highlights the importance of regular radiological monitoring for hospitalized patients with COVID-19. Although SPM is mainly characterized by a benign clinical course and is usually self-limited1,2, as in the case presented here, COVID-19 pneumonia carries the risk of sudden deterioration and, in this setting, occurrence of SPM/SE could lead to further or even fatal cardiorespiratory compromise.
The pathophysiology of SPM lies in alveolar rupture and the ensuing spread of air along the interstitial bronchovascular structures towards the hila and subsequently to the mediastinum. From there, air may track towards the loose anatomic structures of the chest wall and the neck2,9,12,41. This mechanism was first suggested by Macklin in 19391,2, based on experiments in a cat model. The so called ‘Macklin effect’ can nowadays be demonstrated in chest CT imaging as linear collections of air adjacent to the bronchovascular sheaths42. On the basis of the described pathophysiology, pneumothorax only sporadically coexists with SPM/SE6,12. Similarly, in the case presented here, there was only a trace of pneumothorax in the left lung (Figure 3).
Barotrauma induced by positive airway pressures, applied during invasive ventilation, does not appear to be a perquisite for the development of COVID-19 related SPM7. In our case, the patient was on HFNC 60 lpm, which has been estimated to produce a maximum positive end-expiratory pressure (PEEP) of 5 mmHg, only when the patient mouth is closed, and which immediately disappears by opening the mouth43. Therefore, the cause of SPM in our patient can be mainly attributed to the extent and severity of COVID-19, as demonstrated by diffuse bilateral ground glass opacities in chest CT (Figure 3) leading to alveolar damage and rupture, rather than to the unstable low degree of PEEP applied by the HFNC. Moreover, SPM does not occur more frequently in patients on NIV for any etiology, whereas there are some cases of COVID-19 related SPM in patients on NIV12.
CONCLUSION
Further research is warranted to better understand the role of SPM in the prognosis of COVID-19. In many of the cases reported, clinical outcome was poor, most likely due to the severity of COVID-19 and not because of the development of SPM/SE7,12. In our case, the outcome was not fatal, but the patient required longer hospital stay to achieve gradual de-escalation of the oxygen therapy compared to other patients with analogous disease severity. Based on its benign character, it has been proposed that SPM in the course of COVID-19 does not alter the initial prognosis per se but could be a marker of extended disease and poor prognosis12.