INTRODUCTION
Smoking is a well-established risk factor for various forms of cancer (e.g. lung, bladder, and cervical cancer), reproductive issues, menstrual irregularities, respiratory symptoms, and myocardial infarction1,2. Maternal smoking during pregnancy and the postpartum period represents a significant public health challenge, as it is a principal determinant of adverse outcomes for both maternal and neonatal health3,4.
The consumption of tobacco during pregnancy is linked to a spectrum of negative outcomes, including preterm birth, reduced birth weight, intrauterine growth restriction (IUGR), placental abruption, and spontaneous abortions5,6. Secondary exposure to cigarette smoke also jeopardizes the health of neonates, infants, and children, as it exposes them to numerous toxic chemicals and increases their susceptibility to respiratory infections, asthma, and sudden infant death syndrome (SIDS)7,8, as well as neurodevelopmental and behavioral issues9,10. Moreover, such exposure to traditional cigarette smoke can predispose children to adopting smoking behaviors in later life11.
Despite the initial reduction or cessation of smoking upon learning of their pregnancy – a response observed in the majority of women12 – there is a substantial tendency to revert to pre-pregnancy smoking habits post-delivery. The relapse rate into smoking postpartum is alarmingly high, with up to 60% of women resuming smoking, typically within the first trimester after childbirth13. This relapse negatively impacts breastfeeding and is influenced by various factors, including socioeconomic status, level of education14, the presence of depressive symptoms15, and having a partner who smokes12.
Smoking cessation during the puerperium period represents a crucial public health goal, given the well-documented risks of tobacco use on both maternal and neonatal health. Puerperium, the phase following childbirth, offers a unique window of opportunity for interventions due to increased maternal motivation to cease smoking for the sake of the child’s health16,17. However, the effectiveness of smoking cessation interventions during this period remains a subject of ongoing research, necessitating a systematic review to consolidate existing evidence and guide future practices.
The aim of this systematic review was to explore interventions specifically designed for smoking cessation during the postpartum period.
METHODS
Search strategy
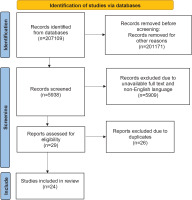
We conducted this systematic review by searching the international literature for studies related to tobacco cessation in the postpartum period. We utilized the electronic databases PubMed and Scopus for the search, adhering to the principles of systematic review methodologies. A comprehensive search strategy was developed using the following keywords in all combinations: ‘tobacco cessation’, ‘smoking cessation’, ‘quitting smoking’, ‘vaping cessation’, and ‘postnatal’, ‘postpartum’, ‘childbearing’, and ‘puerperium’. This search strategy aimed to encompass a wide array of studies pertinent to the cessation of tobacco use among postnatal individuals.
Selection criteria and screening process
A total of 206948 records were initially identified from the PubMed database and 161 records from the Scopus database. We systematically applied several inclusion and exclusion criteria to refine these results:
Temporal limitation: The first inclusion criterion limited the scope of the literature to the last decade, acknowledging the evolution of tobacco cessation methodologies and the relevance of recent studies. This criterion reduced the identified articles to 87666 from PubMed and 96 from Scopus.
Type of study: To ensure the review focused on original research findings, we mandated the inclusion of only primary studies. This filtered the articles down to 5864 from PubMed and 74 from Scopus.
Preliminary screening: Further screening involved a detailed examination of the studies’ titles, abstracts, and samples. The English language was a prerequisite for inclusion to ensure comprehensibility and consistency in the synthesis of findings.
Access to full texts: The final inclusion criterion was the accessibility of full-text articles. This was crucial for an in-depth analysis and verification of the study’s findings, which culminated in the inclusion of 12 articles from PubMed and 17 articles from Scopus.
However, an adjustment was necessary due to the presence of five articles shared by both databases, leading to the selection of a total of 24 unique articles for the systematic review. A flow chart detailing the methodological approach, including the search strategy, inclusion and exclusion criteria, and the screening process, is provided in Figure 1.
RESULTS
This systematic review resulted in 24 articles related to smoking cessation interventions during the puerperium18-41. The articles are summarized in Table 1.
Table 1
Summary of the articles included in the current systematic review
| Title of the article | Authors Year | Country | Type of research | Study sample |
|---|---|---|---|---|
| Quitting smoking before and after pregnancy: Study methods and baseline data prospective cohort study | Cruvinel et al.18 2022 | Kansas Missouri USA | Randomized controlled trial | 62 pregnant/lying-in women |
| Effect of smartphone-based financial incentives on peripartum smoking among pregnant individuals | Kurti et al.19 2022 | USA | Randomized clinical trial | 90 pregnant/lactating women |
| Motivational interviewing telephone counseling to increase postpartum maintenance of abstinence from tobacco | Murphy et al.20 2022 | Rhode Island Massachusetts USA | Randomized controlled trial | 382 pregnant/lactating women |
| Barriers and facilitators to staying smoke-free after having a baby, a qualitative study: Women’s views on support needed to prevent returning to smoking postpartum | Philips et al.21 2021 | United Kingdom | Randomized controlled trial | 26 pregnant/lying-in women |
| Effects of active and/or passive smoking during pregnancy and the postpartum period | Míguez et al.22 2021 | Spain | Randomized controlled trial | 800 pregnant/lactating women |
| Prenatal tobacco smoking is associated with postpartum depression in Japanese pregnant women: The Japan environment and children’s study | Meishan et al.23 2020 | Japan | Randomized controlled trial | 80872 pregnant/lactating women |
| Pediatric office delivery of smoking cessation assistance for breast-feeding mothers | Drehmer et al.24 2020 | Ohio Virginia Tennessee North Carolina Indiana USA | Randomized controlled trial | 2139 breastfeeding women with a child aged ≤1 year |
| Smoking prevalence and secondhand smoke exposure during pregnancy and postpartum - Establishing risks to health and human rights before developing a tailored program for smoking cessation | Frazer et al.25 2020 | Ireland | Randomized controlled trial | 322 pregnant/lactating women |
| Depressive symptoms assessed near the end of pregnancy predict differential response to postpartum smoking relapse prevention intervention | Levine et al.26 2020 | Pittsburgh Pennsylvania USA | Randomized controlled trial | 300 pregnant/lying-in women |
| Description of maternal smoking status before and after pregnancy: A longitudinal, community-based cohort study | Ueda et al.27 2020 | Japan | Randomized controlled trial | 1,220 pregnant/lactating women |
| Influence of puerperal health literacy on tobacco use during pregnancy among Spanish women: A transversal study | Vila-Candel et al.28 2020 | Spain | Randomized controlled trial | 193 lying-in women |
| Smoke-free moms: Financial rewards for smoking cessation by low-income rural pregnant women | Olson et al.29 2019 | New Hampshire USA | Randomized controlled trial | 134 lying-in women |
| Factors associated with post-partum smoking relapse in Taiwan: A trial of smoker’s helpline | Lin et al.30 2019 | Taiwan | Randomized controlled trial | 68 lying-in women |
| Views on and experiences of electronic cigarettes: A qualitative study of women who are pregnant or have recently given birth | Bowker et al.31 2018 | United Kingdom | Qualitative study | 15 pregnant women and 15 lying-in women (n=30) |
| A pilot randomized controlled trial of a phone-based intervention for smoking cessation and relapse prevention in the postpartum period | Coleman-Cowger et al.32 2018 | Baltimore USA | Randomized controlled trial | 128 pregnant/lactating women |
| Self-reported environmental tobacco smoke exposure and avoidance compared with cotinine confirmed tobacco smoke exposure among pregnant women and their infants | Gavarkovs et al.33 2018 | USA | Randomized controlled trial | 147 pregnant/lactating women |
| Examining characteristics associated with quitting smoking during pregnancy and relapse postpartum | Kia et al.34 2018 | Minnesota USA | Randomized controlled trial | 145 pregnant/lactating women |
| Preventing postpartum smoking relapse: A randomized clinical trial | Levine et al.35 2016 | Pittsburgh Pennsylvania USA | Randomized controlled trial | 300 pregnant/lying-in women |
| Efficacy of a nurse-delivered intervention to prevent and delay postpartum return to smoking: The quit-for-two trial | Pollack et al.36 2016 | USA | Randomized controlled trial | 382 pregnant/lactating women |
| Predictors of changes in smoking from third trimester to 9 months postpartum | Shisler et al.37 2016 | Buffalo USA | Randomized controlled trial | 168 pregnant/lactating women |
| Cognitive-behavioral intervention to promote smoking cessation for pregnant and postpartum inner city women | Lee et al.38 2015 | Philadelphia USA | Randomized controlled trial | 277 pregnant/lactating women |
| Predictors of pregnant quitters’ intention to return to smoking postpartum | Pollack et al.39 2015 | USA | Randomized controlled trial | 382 pregnant/lactating women |
| Effect of a family-centered, secondhand smoke intervention to reduce respiratory illness in indigenous infants in Australia and New Zealand: A randomized controlled trial | Walker et al.40 2015 | New Zealand Australia | Randomized controlled trial | 293 lying-in women and their infants |
| Strategies to avoid returning to smoking (STARS): a randomized controlled trial of postpartum smoking relapse prevention interventions | Levine et al.41 2013 | USA | Randomized controlled trial | 300 pregnant/lying-in women |
Risk factors for not quitting or resuming smoking
In many studies of smoking cessation intervention in women, researchers have identified several factors that act as barriers and lead to smoking cessation failure or relapse. These factors are summarized in Table 2.
Table 2
Risk factors for not quitting or resuming smoking
In the study by Kia et al.34, the researchers wanted to examine the characteristics associated with smoking cessation during pregnancy and the relapse during the puerperium. A total of 145 pregnant women participated, of whom 99 (2nd and 3rd trimesters) smoked at least 5 cigarettes per day, and the remaining 46 quit smoking during pregnancy and participated in a study to prevent recurrence in the postpartum period. The results revealed several significant differences in demographics and smoking-related symptoms between the groups and identified specific risk factors associated with postpartum smoking relapse. Of the 46 pregnant women who quit smoking during pregnancy, 50% relapsed during the 12-week postpartum follow-up34.
When compared with pregnant smokers, the pregnant women who quit smoking during pregnancy were found to have a lower level of education, were unmarried, smoked more cigarettes per day before pregnancy, had less motivation to maintain cessation after delivery, started smoking at a young age, made fewer attempts to quit smoking, had more pregnancies and more children, and were more likely to have a partner who smoked28,34.
Pregnant smokers reported feeling overwhelmed by negative emotions and an increased desire to smoke, whereas those with a smoking partner were more likely to continue smoking or experience a relapse during the postpartum period.
In a study22, involving 800 pregnant women from the 1st trimester of pregnancy up to 6 months after delivery, it was found that 37.5% of the sample (n=300) lived with a smoker, and in 84.6% of them, the smoker was their partner. The presence of a smoker in the home environment makes the success of quitting difficult and often leads to the previous smoking situation, while smoking cessation intervention is also required for the partner.
In the study by Pollak et al.39, the researchers recruited 382 pregnant women and followed them up after delivery. The study identified several variables linked to the likelihood of restarting smoking after childbirth. The inclination towards having another child soon and the length of time a woman had stopped smoking were related to her likelihood of resuming smoking. Women less inclined to conceive again soon were found to be more prone to considering restarting smoking, whereas those who had stopped smoking for a longer period were less inclined to revert to smoking. Also, women who said their reason for quitting was temporary rather than permanent were more likely to return; those who said they were not concerned about the effects of smoking on their health were more likely to say they intended to return to smoking compared to women who were concerned; and those who described themselves as non-smokers were more likely not to return to their previous smoking status. The research team determined that while certain factors influencing women’s intentions to revert to smoking remained constant, altering the reasons women cite for quitting smoking could potentially make them more open to shifting their self-identity towards being a ‘non-smoker’ instead of a ‘smoker’ or a ‘woman who is currently not smoking’39.
The longitudinal study by Ueda et al.27 investigated maternal smoking habits from pregnancy to 3 years postpartum. Of the women who participated, about 40% had smoked. Of the 176 pregnant women who reported being ex-smokers at study enrollment, more than 30% returned to their previous smoking status postpartum, and approximately 60% of those who reported being smokers throughout the perinatal period failed to quit. In addition, the researchers found that among the mothers who smoked in postpartum period, more than half were ex-smokers. This result suggests that the prevention of maternal smoking relapse leads to a reduction in total postpartum smoking. Furthermore, the researchers suggested that targeting prevention programs at younger mothers, who are more likely to relapse, would be effective. The study found that the smoking rate among mothers in the rural area of Japan was 10.2% during pregnancy and 37.4% postpartum. Furthermore, the study revealed that participants who smoked both before and after delivery were unable to quit. Finally, younger women were significantly more likely to relapse after delivery than older mothers.
Postpartum depression appears to be associated with smoking, and it occurs more frequently in cases where there is exposure to secondhand smoke during pregnancy. Women who quit smoking <5 years before delivery have an increased risk of postpartum depression compared to those who have never smoked, whereas women who have quitting smoking >5 years do not have an increased risk of postpartum depression compared to those who have never smoked17,23. Assessment of depressive symptoms with tools such as the Edinburgh Postnatal Depression Scale at the end of pregnancy can help determine the success of a postpartum smoking relapse prevention intervention26,30.
Motivation to quit smoking
The effectiveness of a smoking cessation intervention program in the hospital lies in identifying the motivations that will trigger participants to engage in the process, achieve smoking cessation, and protect themselves from the risk of relapse38.
In some studies, a sum of money is given to the participants in the form of a gift card or even cash if they have participated in all the research procedures (e.g. they have answered all the questionnaires, they have responded to the longitudinal meetings, etc.), if proven (e.g. with a saliva cotinine test) that they have stopped smoking, and are maintaining the cessation. The results show that participants, in order to receive the amount of money, are more cooperative in their research obligations and manage to change as well as maintain their smoking status19,29.
Support from the partner (as well as family and friends) is of utmost importance to strengthen the cessation effort and prevent relapse21. In addition, by knowing the dangers of secondhand smoke to infants and children, mothers are more likely to quit smoking and not relapse during the postpartum period or later21. Another motivation is taking care of the mother’s own health, as she has knowledge of the health risks of smoking21 while breastfeeding24.
Breastfeeding was associated with a greater likelihood of abstinence from smoking among those who had smoked at least one cigarette in the past year. The results of the Drehmer et al.24 study show that although the majority of enrolled mothers who smoked while breastfeeding reported that they had recently tried to quit, data collected in routine care screening practices showed that breastfeeding mothers who smoked were not referred to a support setting for smoking cessation. Taken together, this evidence strengthens the rationale for ensuring that all mothers who are breastfeeding or planning to breastfeed are referred to cessation services to be supported and maximize the likelihood that they will use this opportunity not to return to their smoking status and keep the environment they and their children live in free from cigarette smoke.
In another study, it was found that female smokers had no intention to breastfeed. Conversely, those who did not smoke or stopped smoking during the first trimester had increased rates of breastfeeding22. Similar results were found in an earlier longitudinal study by Shisler et al.37 who collected data from 168 women who smoked during pregnancy. The researchers followed the women from their first prenatal appointment to 9 months postpartum. At each assessment interval, maternal interviews were conducted to evaluate breastfeeding, the use of other substances, and smoking by partners as possible factors influencing changes in smoking habits. It was found that while women reduced their tobacco consumption during the prenatal period, nine months postpartum, they significantly increased their smoking. However, women who smoked during pregnancy had a significant predictor of these changes. The more days their infants breastfed, the less they smoked after birth. Prior research reviewed by the team indicated that breastfeeding could postpone the resumption of smoking. This study contributes further by exploring how breastfeeding influences the extent of tobacco use after childbirth among all women, not limited to those who ceased smoking while pregnant. It was found that by the age of 9 months, infants whose mothers breastfed for at least 90 days smoked approximately one-third fewer cigarettes daily compared to non-breastfeeding mothers, and roughly half as many as those who breastfed for 30 to 89 days37.
Smoking cessation intervention programs during the postpartum period
Some of the studies18-20 identified by this systematic review implement a smoking cessation intervention program during the postpartum period using technology [text messages, emails, smartphone applications, videography, telephone counseling lines for smoking cessation, etc.) in order to facilitate participants and urge them to continue the cessation effort by making the necessary meetings (follow-up)] or answering their questions from wherever they were.
Motivational interviewing based on social cognitive theory is a method used in smoking cessation intervention programs during the postpartum period20. Emphasizing the psychosocial factors and individual motivation of the participants, trained health professionals implement a smoking cessation intervention program in puerperium with the ultimate goal of maintaining the home environment free of cigarette smoke and safeguarding the health of the whole family32,36,38.
To certify maternal abstinence from smoking at various time points after delivery (days, weeks, months, and years), many researchers use the measurement of exhaled carbon monoxide (CO) and the detection of salivary cotinine in the mothers or the detection of urine or salivary cotinine in newborns, infants, and children (to certify their exposure to secondhand and thirdhand cigarette smoke and, by extension, their mothers’ smoking)19,26,29,36,41.
Walkers et al.40 conducted an intervention addressing secondhand smoke exposure. The intervention included three home visits in the first three months of the infants’ lives. The control group received ‘usual care’ that included standard management by community health professionals, which ranged from brief cessation advice to the provision of cessation treatment. In the intervention group, all mothers who smoked (and family members who lived with the mother and child and were involved in their care and who were smokers) received usual care and cognitive behavioral intervention about the risks of secondhand smoke exposure for children, commitment to smoking restrictions at home and in the car, positive role modeling, and strategies for overcoming barriers to making smoking behavior change. Additionally, the intervention group provided brief advice to smokers interested in quitting, offered free nicotine replacement therapy (NRT), and referred them to a support line. Both groups received brief health promotion messages (focusing on immunization, breastfeeding, and safe infant sleep) at the start of the study and at 4 and 12 months of the infants’ lives.
This trial40 examined whether a family intervention to protect against secondhand smoke exposure that focused primarily on infant health, as opposed to smoking cessation in adults, had any effect on the incidence of respiratory disease and the need to seek medical help. Over a 12-month period, no effect was found. Although mothers reported that infants had low secondhand smoke exposure, mean infant urine cotinine values at baseline and 4 months of age were consistent with environmental exposure to smoke. This may be due to infant exposure to tobacco products through breast milk or unreported or underestimated secondary exposure to tobacco smoke from the mother. Furthermore, the intervention had no effect on the incidence of respiratory disease in children requiring hospitalization or seeking medical assistance for treatment. The above findings suggest that keeping the family smoke-free at home and in the car is not enough to protect newborns, infants, and children from secondhand smoke exposure. Family members who smoke should be encouraged to quit at the time of conception and maintain their cessation after delivery and mothers should be discouraged from smoking while breastfeeding40.
Another study35 evaluated the effectiveness of two different approaches to prevent postpartum smoking relapse. The researchers recruited and randomly divided 300 pregnant women who had recently quit smoking into two groups shortly before delivery for this purpose. The first group (the control group) received simple smoking cessation support, and the second group (the intervention group) received enhanced cognitive-behavioral intervention focused on psychosocial factors (postpartum concerns related to mood, stress, and body weight). The program was administered using both telephone consultations and face-to-face meetings, beginning just before childbirth and extending up to 24 weeks after birth. Assessments were conducted with participants before the birth and then again at 12, 24, and 52 weeks following childbirth. Improvements in mood and stress levels post-birth were reported across all participants, with a decrease in symptoms of depression and stress linked to continued non-smoking. Conversely, those exhibiting greater depressive symptoms and increased stress were found to have a higher tendency to revert to smoking35.
Gavarkovs et al.31 conducted a study to investigate the relationship between self-reported exposure to cigarette smoke and its avoidance by detecting salivary cotinine in non-smoking pregnant women, the relationship between self-reported avoidance of cigarette smoke and detection of salivary cotinine in postpartum non-smoking mothers, and the relationship between infant exposure to cigarette smoke and maternal avoidance as recorded by measurement of infant salivary cotinine. The researchers categorized the study participants as smokers or non-smokers, measured the salivary cotinine of pregnant women at 32 weeks’ gestation, and recorded the salivary cotinine of mothers and infants at 6 months postpartum, all while recording exposure to or avoidance of cigarette smoke. The findings of the study indicate that using self-reported data on tobacco smoke exposure and behaviors to avoid it could serve as a suitable proxy for evaluating such exposure in women during and after pregnancy, as well as in their newborns. The study also shows that reducing exposure to tobacco smoke correlates with lower concentrations of salivary cotinine, a marker of nicotine intake, during pregnancy and in the infant’s environment. However, it is noted that self-reported exposure to cigarette smoke correlates with salivary cotinine levels only in the postpartum period, not during pregnancy. Furthermore, the exposure risk to tobacco smoke for pregnant women or infants can be influenced by the smoking habits of their family, friends, and caregivers who are not family members, highlighting the importance of evaluating exposure in various environments, not just at home. The observation that increased salivary cotinine levels correspond with a greater number of reported exposure sites, underscores the value of examining both workplace and residential settings to better assess exposure risks31.
Varenicline, nicotine replacement therapy (NRT) and electronic cigarette
Varenicline, nicotine replacement therapy, and more recently, the electronic cigarette, are used as means to reduce or stop smoking, but also to prevent the recurrence of herpes. Bowker et al.33 conducted semi-structured telephone interviews with pregnant and lying-in women. The use of e-cigarettes was valued as a positive choice by some, as they considered it less harmful and a useful tool to achieve smoking cessation. However, due to social stigma, some participants stated that they felt uncomfortable using e-cigarettes in public, especially during pregnancy, and at the same time expressed concerns about the health safety of using e-cigarettes and nicotine dependence, and for these reasons they showed a preference for NRT. In one more study21 carried out in the following years, the results were similar: several of the participants expressed concern and did not wish to use any of the above methods as they felt that their nicotine addiction would continue. However, they appeared more adaptable if a health professional recommended the above methods to them. Thus, the researchers agreed that health professionals should provide informed and consistent information and advice about safety and addiction, as well as consider the influence of social stigma.
Role of health professionals in smoking cessation in puerperium
Health professionals dealing with the perinatal period, while asking about tobacco use in the early stages of pregnancy during history-taking, do not provide systematic counseling about smoking cessation throughout the perinatal period, and women who want to try to change their smoking habit do not find the appropriate supporting framework18.
Support for smoking cessation and relapse prevention from health professionals should continue into the postpartum period and support not only the woman but the whole family21. A similar observation was made by Frazer et al.25 who highlighted the importance of developing smoking cessation programs specifically for the perinatal period in order to ensure the child’s human right to health and well-being, as it will be protected from secondhand smoke exposure exposed through his smoking parents. Moreover, health professionals can play a key role in promoting maternal and child health through actions to promote breastfeeding, smoking cessation, and relapse prevention22.
Meta-analysis
If an article fulfilled the subsequent population, intervention, comparison, outcomes, and study (PICOS) design criteria, it was eligible for inclusion in the present meta-analysis: 1) Population: limited to lying-in women; 2) Intervention: any type of intervention for smoking cessation during the puerperium versus standard of care; 3) Comparison: studies comparing the outcome between lying-women who received any type of intervention for smoking cessation during the puerperium versus lying-in women who received standard care; 4) Outcome: Abstinence from smoking at six months after birth; and 5) Studies. These are already included in Table 2.
All analyses were carried out using Review Manager Software (RevMan), version 5.4. Heterogeneity across trials was identified using I2 statistics; considering I2>50% as high heterogeneity, a meta-analysis was conducted using a random-effect model according to the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1.0)42. For discontinuous variables, odds ratios (OR) with 95% CIs were applied for the assessment. A p<0.05 was considered to indicate a statistically significant difference.
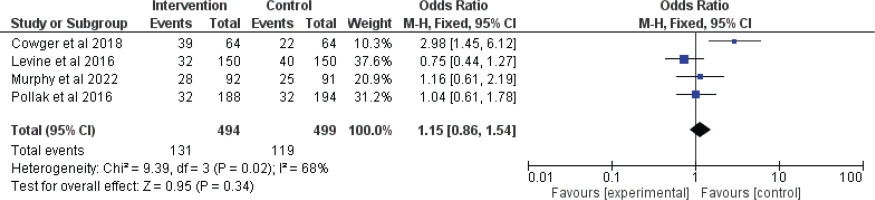
In total, four articles20,32,35,36 met the eligibility criteria. The total number of participants was 993 (494 in intervention group and 499 in control group). All the studies were randomized control trials. No significant difference was found between the intervention and controls groups regarding the abstinence from smoking at six months (OR=1.15; 95% CI: 0.86–1.54; p=0.34), with heterogeneity (p=0.02 and I2=68%) (Figure 2).
DISCUSSION
This review highlights several risk factors for smoking cessation failure or relapse in the postpartum period, including psychological factors (anxiety, postpartum depression, and negative emotions), social factors (alcohol consumption, the presence of a smoking partner, and returning to work), and behavioral factors (strong cravings for cigarettes and low motivation for cessation). These findings are consistent with the findings from another study43, who identified mental health issues and the partner’s smoking status as significant predictors of postpartum smoking relapse. Similarly, Chamberlain et al.44 emphasized the role of socioeconomic factors, such as lower level of education and income, in smoking persistence postpartum, aligning with the observations regarding education level and income in the current review.
The participants’ motivations significantly influence the effectiveness of smoking cessation interventions. We identified economic incentives, partner support, safeguarding the health of the new family member, personal health concerns, and breastfeeding as key motivators. Higgins et al.45 corroborate this, finding that financial incentives significantly enhance smoking cessation rates among pregnant and postpartum women45. The review observed the protective role of breastfeeding in sustaining smoking abstinence, which aligns with the findings of Giglia et al.46 who suggested that breastfeeding can serve as a strong motivator for mothers to quit smoking and prevent relapse. Our review concludes that the relationship between breastfeeding and maintaining abstinence is positive, as breastfeeding has great benefits for the health of the mother and the child, it can play a protective role and inhibit relapse, since it promotes abstinence.
The reviewed studies underscore the potential of hospital-based intervention programs, employing technology and motivational interviewing, to facilitate smoking cessation. These findings resonate with the work of Naughton et al.47 who demonstrated the effectiveness of a proactive approach, including texting support, in reducing smoking rates among pregnant women. The emphasis on targeting younger mothers and the significance of family-wide cessation efforts find parallels in the study by Lumley et al.48, which suggested tailoring interventions to address the specific needs and circumstances of the postpartum population.
The systematic review suggests that health professionals often miss opportunities to provide systematic counseling throughout the perinatal period. This observation echoes the concerns raised by Flemming et al.49 who noted a gap in healthcare providers’ engagement with smoking cessation interventions during and after pregnancy. The findings of Fang et al.50 who advocated for sustained intervention strategies that extend into the postpartum period to address the risk of relapse, support the need for continuous support, as identified in the review. In addition, Notley et al.51 found that there is a need for support the lying-in women to prevent postpartum smoking relapse. The women reported that they had received support to stop smoking during pregnancy, but this did not happen after the birth, even though they wanted it and considered it necessary. Postpartum support should be tailored to the individual needs of women who are more vulnerable to recurrence and delivered by a qualified and trained health professional, preferably a midwife or health visitor, as reported by the participants themselves. In addition, the participants had a positive opinion about being given an information leaflet on relapse prevention at the surgery, as well as positive comments about being able to have electronic access to digital material with information on smoking cessation and relapse prevention whenever they wish or have time in their daily life. They even stated that it was important to them that all information given to them be based on recent research data and be scientifically documented51.
In addition to the findings discussed, it is important to consider potential factors influencing smoking relapse, such as baseline mental health status and fear of weight gain. Lying-women with poorer mental health at baseline may have higher relapse rates due to increased stress and anxiety, which can trigger smoking as a coping mechanism41. Additionally, the fear of weight gain, which is common among lying-in women attempting to quit smoking, may also contribute to relapse52. These psychological factors should be taken into account when designing and implementing smoking cessation interventions to improve their effectiveness and address individual needs comprehensively.
Regarding the meta-analysis, the overall non-significant result (OR=1.15; p=0.34) suggests that, when combining the results of these four studies, the intervention does not significantly increase smoking abstinence at six months compared to the control. The significant heterogeneity indicates that there may be important differences between the studies that could affect the results. Possible sources of heterogeneity could include differences in intervention delivery, population characteristics, or study settings. The significant effect observed in Coleman-Cowger et al.32, highlights that some interventions might be effective under specific conditions or in certain populations, but this effect was not consistently observed across all studies.
Limitations
This systematic review has some potential limitations. The search strategy might have inadvertently omitted relevant studies due to the specificity of keywords, databases searched, or language restrictions. This limitation could result in a selection bias, ignoring potentially important findings from studies published in languages other than English or indexed in databases that were not included in the search. The included studies might vary significantly in design, methods, and quality. This heterogeneity can complicate the synthesis of findings and limit the ability to draw generalized conclusions. Differences in population demographics, intervention specifics, and outcome measures across studies can introduce variability that challenges the synthesis of data. Publication bias may affect the review, as studies with positive outcomes tend to receive more publication than those with negative or inconclusive results. This could skew the review’s findings towards more optimistic interpretations of the efficacy of smoking cessation interventions. Many studies included in the review may not have long-term follow-up data, limiting insights into the sustainability of smoking cessation over time. The puerperium is a dynamic period, and the long-term effectiveness of interventions is crucial for understanding their true impact. Studies likely varied and did not uniformly report details regarding the implementation of interventions, fidelity to intervention protocols, and participants’ adherence. This variability can make it difficult to identify which components of interventions are most effective. The studies included in the review might not have adequately controlled for all relevant confounding factors, such as participants’ previous attempts to quit smoking, psychiatric comorbidity, or substance use. This could affect the attribution of outcomes to the interventions assessed.
Implications
While the systematic review sheds light on various aspects of smoking cessation in the puerperium, it also highlights several gaps in the current body of knowledge. There is a need for more research on the long-term effectiveness of different intervention strategies, particularly in diverse populations and settings. The role of e-cigarettes and nicotine replacement therapy in smoking cessation during the puerperium remains contentious and warrants further investigation, considering the mixed perceptions among participants regarding their safety and efficacy. Additionally, the impact of smoking cessation on maternal and infant health outcomes beyond the immediate postpartum period deserves closer examination.
CONCLUSIONS
This systematic review underscores the critical need for targeted interventions to address smoking cessation during the postpartum period. Despite initial reductions in smoking rates during pregnancy, high relapse rates post-delivery highlight the complex challenges faced by new mothers. Factors such as socioeconomic status, a partner’s smoking status, and depressive symptoms, significantly influence smoking behavior during this vulnerable period. Motivations for cessation include concerns for infant health, support from partners, and financial incentives. Interventions employing technology-based approaches, motivational interviewing, and cognitive-behavioral strategies show promise in supporting smoking cessation efforts. However, ongoing support from health professionals, particularly during the postpartum period, is crucial to sustain cessation and prevent relapse. Additionally, interventions targeting family members’ smoking behaviors and emphasizing the health benefits of breastfeeding are essential components of comprehensive cessation programs. Verification methods such as exhaled carbon monoxide measurement and salivary cotinine detection play a vital role in monitoring cessation outcomes. Varenicline, nicotine replacement therapy, and electronic cigarettes offer potential as cessation aids, although attitudes toward their use vary among participants. Overall, this review highlights the importance of tailored interventions and continued support from health professionals to address smoking cessation in the postpartum period effectively, ultimately safeguarding the health of both mothers and infants. We need further research to refine and optimize cessation strategies tailored to the unique needs of postpartum women.