INTRODUCTION
During the past two decades, two major global pandemics emerged from the mutation and uncontrolled transmission of two already well-known viruses. The first appeared in the USA in April 2009 due to (H1N1) pdm09, a subtype of the influenza virus family and resulted in 18036 deaths within one year1. The second is caused by SARS coronavirus 2 (SARS-CoV-2) and first appeared in Wuhan, China in December 2019, already counting millions of deaths2.
Both H1N1 and SARS-CoV-2, two primarily respiratory viruses, resulted in many cases of patients with Acute Respiratory Distress Syndrome (ARDS). ARDS according to the Berlin Criteria (2012), is defined as respiratory distress of acute onset (within a week of a clinical incident), with diffuse lung infiltrates on chest X-ray, without underlying cardiac failure or abnormally positive fluid balance. The activation and dysregulation of multiple pathways of injury and inflammation, including coagulation, are the main constituents of ARDS pathophysiology3. Also, partial pressure of oxygen in the arterial blood to the fraction of inspired oxygen concentration PaO2/FiO2 should be ≤3004. Causes of ARDS are divided into pulmonary, which could be respiratory tract infection or lung injury, and extrapulmonary, like burns, prior non-thoracic operation, extrapulmonary infection etc. In terms of hospital mortality, it is 34.9% for mild disease, 40.3% for moderate, and 50.4% for severe ARDS5. Finally, ARDS is the primary cause of Intensive Care Unit (ICU) admissions.
According to the World Health Organization (WHO), risk factors for worse prognosis for H1N1 virus disease are young age, pregnancy, old age and comorbidity6. On the other hand, risk factors for SARS-CoV-2 associated with severe disease are still under investigation. So far, it seems that old age and comorbidity are also risk factors for ICU admissions. However, ARDS and death by SARS-CoV-2 have been described in younger patients, children, and parturients7.
Parturients are considered a high-risk population and are often susceptible to various severe forms of infection such as pneumonia, pyelonephritis, chorioamnionitis and others. The rare occurrence of ARDS in pregnancy makes it relatively difficult to study in a systematic way. Nonetheless, there are a few systematic reviews on the matter. The largest, so far, gathered data from 2006–2012 from Nationwide Inpatient Samples, found the incidence of ARDS in pregnancy to be 0.05%, with the mean age of patients being 28.7 years. Mean hospital mortality was found to be 9%, while in patients undergoing prolonged mechanical ventilation (>96 h) it was 14%. According to Rush et al.8, improved survival was related to improved mechanical ventilation techniques among parturients in comparison to the past, and younger patients age 8. Muthu et al.9 compared ARDS epidemiology in pregnant and non-pregnant women from 2009 to 2016 and found that maternal mortality was 18% with no difference between trimesters, while fetal mortality was 37%. Mortality in non-pregnant women was 20%. Additionally, age in pregnant patients was significantly lower than in non-pregnant women (25 vs 32 years)9.
The goal of this review is to examine the vulnerability of pregnant women to H1N1 and SARS-CoV-2 infection. Moreover, we investigate the incidence of ARDS and its consequences on perinatal outcomes, as well as similarities and differences between those concerning epidemiology, prognosis, and outcome.
METHODS
In May 2020, we started conducting the literature search on PubMed and Embase, and on PubMed, Embase and Cochrane databases, for studies published between database inception and March 2022, for SARS-CoV-2 and H1N1 viruses, respectively.
For COVID-19, search keywords were: SARS-CoV-2, COVID-19, Pregnancy, ICU, and ARDS. Two researchers (PD and PT) independently screened articles, by reviewing titles and continued the initial screening process, by reviewing the abstracts of the remaining articles. They discussed inconsistencies and in cases of disagreement they consulted a third reviewer, to obtain a consensus. Only articles in English and with human participants were accepted. No case reports were accepted, only a limited number of cases series. No randomized-control trials were found, so research was focused on prospective and retrospective cohort studies. Abstracts and congress presentations were not included. The final qualitative screening step was again performed by both the reviewers independently and the last studies were excluded, due to being not completely relevant. The main outcome of the study was severe cases of COVID-19 (the respiratory disease caused by the strain of coronavirus SARS-CoV-2) in pregnancy defined by ICU admission. Admission to ICU and ARDS were two independent variables and they were examined separately. In the ICU period, days of mechanical ventilation were assessed. Moreover, use of ECMO and its outcomes were studied as a support method for severe ARDS. Neonatal outcome data were extracted from all mothers admitted to the ICU. The team also conducted a ‘pearl’ search in the last months to identify additional studies added in the databases, by searching the citation lists of publications eligible for full-text review.
For influenza A/H1N1, the search was performed by using the keywords: H1N1, Pregnancy, ICU, and ARDS. The search was focused on influenza A/H1N1 and not on other types of influenza. Again, the same screening pattern was followed. The main focus of the study was severe cases of H1N1 in pregnancy, defined as admitted to ICU. The final qualitative screening step was performed by both the reviewers independently and the last studies were excluded due to being not completely relevant or having poor study quality. Neonatal outcome data were examined from all mothers admitted to ICU and not necessarily due to ARDS. Finally, a reverse ‘snowball’ search was added in the protocol, by using the reference lists of publications eligible for full-text review.
RESULTS
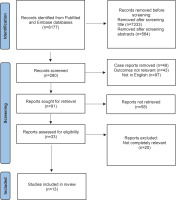
Results for COVID-19 are presented in Figure 1. A total of 13 studies were included examining maternal morbidity and mortality, maternal ARDS caused by SARS-CoV-2, and neonatal outcomes. Data on maternal morbidity are given from 5 studies. Data on maternal SARS-CoV-2 induced ARDS are from 6 studies (Table 1), and neonatal outcome from 5 studies.
Table 1
SARS-CoV-2 induced ARDS
| First author | Year of publication | Study type | Number of women admitted to ICU | Number of women with ARDS | Number of women on ECMO | Days of mechanical ventilation | Mortality n (%) |
|---|---|---|---|---|---|---|---|
| Marwal29 | 2021 | Case series | 4 | 4 | 0 | 1 | 0 |
| Scheler26 | 2021 | Cross-sectional | 5660 | 5660 | Unknown | Unknown | 377 (7.8) |
| Bamasood27 | 2022 | Retrospective cohort | 18 | 10 | 10 | 3 | 1 (5.0) |
| WAMP31 | 2020 | Retrospective cohort | 43 | 7 | 2 | Unknown | 3 |
| Pierce-Williams28 | 2020 | Retrospective cohort | 20 | 14 | 0 | 3 | 0 |
| Yan30 | 2020 | Retrospective cohort | 6 | 6 | 1 | Unknown | 0 |
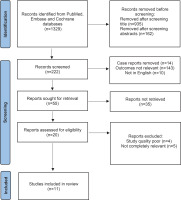
Results for influenza A/H1N1 are presented in Figure 2. A total of 11 studies were included examining maternal morbidity and mortality, maternal ARDS caused by H1N1, and neonatal outcomes. Data on maternal morbidity and mortality are from 6 studies. Data on maternal H1N1 induced ARDS are from 4 studies (Table 2), and neonatal outcome from 5 studies.
Table 2
H1N1 induced ARDS
| First author | Year of publication | Study type | Number of women admitted to ICU | Number of women with ARDS | Number of women on ECMO | Days of mechanical ventilation | Mortality n (%) |
|---|---|---|---|---|---|---|---|
| Anzic20 | 2011 | Retrospective cohort | 64 | 44 | 9 | 9 | 7 (10.9) |
| Nair22 | 2011 | Retrospective cohort | 12 | 12 | 9 | 25 | 4 (33.33) |
| Handyal23 | 2015 | Case series | 3 | 3 | 0 | 12 | 1 (33.33) |
| Dubar24 | 2010 | Prospective cohort | 40 | 20 | 11 | 13 | 3 (7.5) |
No eligible studies emerged by using the references or citations of those already examined, in the time period we conducted the search.
Maternal morbidity and mortality
According to the data gathered, maternal morbidity and mortality in H1N1 was more prominent within the second and third trimester10-13. Worse prognosis was associated with obesity and history of asthma9. Another common component between the studies was that women who eventually died were administered oseltamivir relatively late (>48 h) in contrast to women who survived, to which antiviral agents were administered within 48 h from the onset of symptoms 10-15 (Table 3). Regarding parturients with COVID-19, high morbidity and mortality rates were associated with various comorbidities, with the most important being obesity and hypertensive disorders of pregnancy. Moreover, higher maternal age (>30 years) was another important risk factor, while that was not the case in H1N1 positive parturients. Overall death rate was higher in the H1N1 pandemic compared to COVID-19 pandemic in the pregnant population 16-19 (Table 4). A significant difference between the two viruses is that in the case of influenza, a specific antiviral agent was discovered and administered as it seemed to improve the prognosis in parturients, as it did for the general population, while for COVID-19, a specific antiviral agent was not found by that time16-20.
Table 3
Maternal morbidity and mortality attributed to H1N1
| First author | Year of publication | Study type | Country | Number of women | Hospitalizations n (%) | Deaths n (%) |
|---|---|---|---|---|---|---|
| Da Silva10 | 2014 | Prospective cohort | Brazil | 163 | 70 (43) | 18 (11) |
| Doyle11 | 2013 | Retrospective cohort | USA | 187 | 163 (87) | 8 (4) |
| Jamieson12 | 2009 | Prospective cohort | USA | 34 | 14 (41) | 1 (2.9) |
| Siston13 | 2010 | Prospective cohort | USA | 788 | 115 (22.6) | 30 (3.8) |
| Al Husban14 | 2019 | Retrospective cohort | Jordan | 27 | 19 (70) | 1 (3) |
| Kim15 | 2010 | Retrospective cohort | Korea | 150 | 12 (8) | 0 (0) |
Table 4
Maternal morbidity and mortality attributed to COVID-19
| First author | Year of publication | Study type | Country | Number of women | Hospitalizations n (%) | Deaths n (%) |
|---|---|---|---|---|---|---|
| Epelboin16 | 2021 | Retrospective national cohort | France | 874 | 874 | 2 (0.23) |
| Qeadan17 | 2021 | Retrospective cohort | USA | 1609 | 974 (60) | 4 (0.2) |
| Villar18 | 2021 | Retrospective multinational cohort | 706 | 706 | 11 (1.6) | |
| Asalkar19 | 2021 | Cross-sectional | India | 871 | 871 | 9 (1.03) |
ARDS
ARDS caused by H1N1 infection was more common in the third trimester and post-partum 21-24. The average days of mechanical ventilation varied from 8 to 25 days21-24. Better outcomes were associated with early onset antiviral agent administration. Prone position was used in one study in some patients23. ECMO was used in some patients who had severe ARDS from which 66% survived22. Dubar et al.24 noted that 25% of women developed secondary infection (Table 2).
Secondary bacterial infection was not extensively studied in the SARS-CoV-2 cases during pregnancy, nevertheless, according to a review by Lachana et al.25, risk factors for secondary infection admitted to ICU is hospitalization for >48 h in the ICU, COVID-19 disease severity, number of lymphocytes (0.7×109/L) and lower values of PaO2/FiO2 ratio. ARDS induced by COVID-19 was also more common in the third trimester and post-partum 26-28. There was no anti-viral agent that seemed to improve maternal outcomes; however, mortality rate could not be assessed since results were published prior to maternal discharge or death20. Ten patients were placed on ECMO and 9 survived 27. Thus, present evidence shows that ECMO had better results in survival in ARDS induced by SARS-CoV-2 compared to H1N1. Nevertheless, it is not clear whether this is related to the experience gained in the last two years. The average ventilator days for parturients with ARDS caused by COVID-19, was 1 to 3 days (Table 1)26-31.
Thus, one major difference between H1N1 and COVID-19 is the average of ventilator days was much higher in the H1N1 induced ARDS. Lung mechanics were only assessed in one study, that of Bamasood et al. 27, and those patients were all placed on ECMO, and had Murray scores of 3–4, which indicate severe ARDS (Murray score includes 4 criteria for the development of ALI/ARDS from 0 to 4 according to the severity of the condition). The mean value of their static compliance (Cstat) was 23.5 mL/cmH2O (stiff lung) and the average positive end expiratory pressure (PEEP) placed 16.4 cmH2O27.
Perinatal outcomes
Perinatal outcomes were assessed only in women admitted to the ICU. Neonatal outcomes, however, were assessed in COVID-19 and H1N1 infected women regardless of disease severity. Neonatal outcomes of women in those groups admitted to the ICU are still very heterogeneous (Tables 5 and 6). In general, neonates born from H1N1 positive mothers were premature, delivered by cesarean section and were admitted to the neonatal ICU (NICU) to a large extent due to prematurity. Even in patients who had to be placed on ECMO treatment, 7 livebirths were after the initiation of ECMO treatment, and from 12 neonates delivered, 10 were alive despite the extreme prematurity (mean gestational age of 31 weeks) 22 . Data from the Center for Disease Control (CDC) showed that from a cohort of 168 women being admitted to the ICU, neonatal survival was 88%32. Concerning neonatal outcomes of SARS-CoV-2 positive mothers admitted to the ICU, results were slightly better than H1N133,34. From the study in which women were placed on ECMO, 9 of 10 neonates were liveborn and the one undelivered fetus was of the deceased mother 31 (Table 1). In another study by Hantoushzadeh et al.35 in which 7 out of 9 women died, there were 4 liveborn neonates (Table 6). In general, the incidence of preterm delivery due to maternal deterioration, cesarean delivery and admission to NICU was high. Nevertheless, adverse neonatal outcomes in terms of mortality were not very common in COVID-19 cases. From the present data, results on perinatal outcomes between the two groups are comparable with slightly better survival being in the COVID-19 group.
Table 5
Perinatal outcomes of ICU admitted women (H1N1)
| First author | Year of publication | Study type | Number of women admitted to ICU | Total number of neonates born | Number of liveborn neonates |
|---|---|---|---|---|---|
| Dubar24 | 2010 | Prospective cohort | 40 | 33 | 33 |
| Nair22 | 2011 | Retrospective cohort | 12 | 12 | 10 |
| ANZIC21 | 2011 | Retrospective cohort | 64 | 60 | 56 |
| AI-Husban14 | 2019 | Retrospective cohort | 2 | 2 | 2 |
| CDC32 | 2009–2010 | Retrospective cohort | 168 | 160 | 148 |
Table 6
Perinatal outcomes of ICU admitted women (COVID-19)
| First author | Year of publication | Study type | Number of women admitted to ICU | Total number of neonates born | Number of liveborn neonates |
|---|---|---|---|---|---|
| Pierce-William38 | 2020 | Retrospective cohort | 20 | 18 | 18 |
| Bamasood27 | 2022 | Retrospective cohort | 10 (ECMO) | 9 | 9 |
| Dileep34 | 2022 | Retrospective cohort | Unknown 53 (severe disease) | 53 | 53 |
| Hantoushzadeh35 | 2020 | Case series | 9 | 5 | 4 |
| Asalkar19 | 2021 | Cross-sectional | Unknown (9 deceased) | 3 | 3 |
Vaccination status and efficiency was not assessed to a great extent, since many studies were performed prior to approval of vaccination to pregnant women for both H1N1 and SARS-CoV- 2. Vaccination was found to reduce the incidence of polymerase chain reaction (PCR) proven maternal infection by 50% in H1N1 infection 36 . The risk of congenital malformations, preterm birth, and miscarriage was not found to be increased compared to non-vaccinated individuals 37. However, evidence as to whether the disease impact to neonate is reduced, is still inadequate 38. As far as neonatal outcomes are concerned, various studies have demonstrated that vaccination reduces hospitalization in the first months of life, which is attributed to both placental crossing of maternal antibodies and to possible decreased disease severity 38. According to Pratama et al.39, mRNA vaccines for immunization against COVID-19 potentially reduces the risk of SARS-CoV-2 infection in pregnancy. Antibody placental transfer was reported since neonates of vaccinated mothers had higher levels of IgG antibodies. Finally, it is worth mentioning that even though vaccination against influenza has not scientifically proved a reduction in COVID-19 severity, according to a large cross-sectional epidemiological study performed in Greece by Kopsidas et al.40, people who regarded themselves more susceptible to severe COVID-19 infection were more likely to get vaccinated against influenza, even if they had not been vaccinated against the virus in the past.
DISCUSSION
Multiple physiological changes occur during pregnancy which, admittedly, change the state of women making them high-risk for increased severity of infections, and certain autoimmune diseases. One of those changes is weight gain. The higher the pre-pregnant body mass index (BMI) the lower the weight encouraged to be gained in order for maternal and fetal well-being 41. Nevertheless, remaining within the recommended limits is not very common. In fact, more than 50% of parturients in the US are obese and 8% are extremely obese (BMI >40 kg/m2) 42. Regarding the cardiorespiratory system, there is a progressive increase in total blood volume and cardiac output, reduction of systemic vascular resistance and pulmonary vascular resistance.
Moreover, there is a decrease in vascular colloid pressure, which leads to pulmonary edema formation susceptibility. Oxygen consumption is increased by approximately 15% and minute ventilation by approximately 40–50% 43. The main mechanical changes parturients undergo are diaphragmatic elevation due to abdominal distention leading to reduction of functional residual capacity (FRC) and extension of the anteroposterior diameter of the chest wall, caused by the relaxation of rib fibrocartilage joints 41. Consequently, vital capacity is generally sustained throughout pregnancy so that it matches increased oxygen consumption41. Therefore, previous assumptions that dyspnea of pregnancy is attributed to declining vital capacity are probably false. In fact, a possible explanation for this phenomenon is a disproportionate increase in minute ventilation needs and actual increase in vital capacity41. Additionally, some evidence has shown that even airway resistance increases during pregnancy44. All the previous changes occur in order to increase the delivery of oxygen to peripheral tissues and particularly to the fetus.
Evidence shows that oxygen consumption increases during pregnancy45. Thus, deterioration of parturients with community acquired pneumonia (CAP) that would not have the same effect had not they been pregnant, is partly explained. Furthermore, immunological changes are also crucial for infection in pregnancy. It has been shown that there is increased leukocyte number with increased number or polymorphonuclear cells (PMCs), and particularly immature granulocytes41. It has also been shown that there is impaired chemotaxis gradually, with a peak on the 26th week of gestation, partly explaining higher incidence of symptomatic infections on the second, and mainly third, trimester of pregnancy46.
Evidence is rich concerning epidemiological data of both infections and particularly COVID-19 disease. Nevertheless, data concerning severe disease and ARDS are not yet adequate.
Pregnancy outcomes from the COVID-19 pandemic have been widely studied during the past two years. According to Hantoushzadeh et al.35 who performed a systematic review and meta-analysis on perinatal outcomes of COVID-19 infection, the most common comorbidities in positive parturients were hypertension and diabetes, while among the non-obstetric comorbidities was asthma. Furthermore, concerning perinatal outcomes, around 30% of parturients experienced pre-term delivery. Premature rupture of membranes and fetal distress were developed by 2% of the studied cases. Mean birth weight was 2855 g and prevalence of small-for-gestational age was 17.4%. Apgar scores were 8.8 and 9.2 at 1 and 5 min, respectively47.
Furthermore, Pathirathna et al.48 performed a systematic review (21 observational studies) on perinatal outcomes in COVID-19 infected parturients. Maternal death was found to be significantly increased in infected individuals (p<0.05), and pre-eclampsia was found to be significantly incident in infected parturients (p<0.05). Moreover, cesarean section was found to be significantly increased as a method of delivery in infected patients compared to non-infected individuals (p<0.05). No statistical significance was found in miscarriages/abortions, and in premature rupture of membranes (PROM), between infected and non-infected individuals. Regarding fetal outcomes, fetal distress was found to be increased in infected women (p<0.05) but no increase in neonatal death was found. Preterm birth, low birth weight, APGAR score of <7, and admissions to the NICU, were all found to be increased in infected individuals (p<0.05). Neonatal death was not found to be statistically significant48.
Recent data have been gathered mainly after the 2009 Influenza A/H1N1 pdm09 and have variable results. Hansen et al.49 gathered data from July 2008 to May 2010 from a sample of 109015 pregnancies, from which 959 (0.9%) were diagnosed with H1N1. Firstly, increased incidence of influenza related adverse outcomes were associated in parturients suffering from asthma (8.1% vs 3.9%), neurologic disease (3.6% to 0.9%) or obesity (29.8% to 22%)49. Moreover, women who were diagnosed with H1N1 or seasonal influenza were commonly non-Caucasian and aged 20–29 years49. Hospitalization within 30-days of diagnosis was more common in parturients infected with H1N1 (15%) compared to seasonal influenza virus (4%). Antiviral treatment was administered in 553 parturients infected with H1N1, early (within 2 days of diagnosis), and in 34 women late (3–7 days after diagnosis), and the rate of 30-day hospitalization was three times higher in women who received late antiviral treatment49.
A systematic review performed by Meijer et al. (100 studies-observational and randomized control mostly) concerning perinatal outcomes of H1N1 infection50, found that women from second trimester onwards are at increased risk of hospitalization. Up to 55% was found to have underlying conditions. The range of hospitalized women classified as severe cases was 2–64%, and median duration of hospitalization was 3–9 days. Maternal mortality rate ranged from 0–33%50. Fell et al.51 assessed the risk of perinatal abnormalities in a systematic review (21 comparative studies) and found that parturients not suffering from influenza had a risk of preterm birth ranging from 5.2% to 11.5%, while women who were positive to influenza A/H1N1 pdm had an incidence ranging from 4 to 25.8%. The range of results is relatively wide due to the fact that studies were heterogeneous, especially in disease severity. Among women who had severe disease, the risk of preterm birth was found to be 24%.
Nonetheless, in epidemiological studies of parturients of variable disease severity, no significant risk of preterm birth was found51. The incidence of fetal death, miscarriages/abortions and PROM were not meaningfully interpreted since evidence was very heterogeneous51. Another national cohort study, assessing the same matter38, found that the stillbirth rate in 2009/H1N1 pandemic in UK was 27 (95% CI: 11–56) per 1000, showing an association between H1N1 infection and perinatal mortality. Moreover, H1N1 positive parturients had higher incidence of delivering preterm (<37 weeks of gestation) and 72% of those deliveries were by cesarean section due to deteriorated maternal state. Mean birth weight was lower in neonates of H1N1 positive mothers compared to negative mothers. Nevertheless, this was attributed to lower gestational age of those neonates. Congenital abnormalities were found to be 32 (95% CI: 14–62) per 1000. Incidence of neonatal NICU admission was 33%38.
All the above data referred mainly to the general effects of SARS-CoV-2 and H1N1 in the pregnant population. Nonetheless, the goal of this study was to assess the effects of severe disease on pregnancy. Data about this are still very inhomogeneous both in H1N1 and SARS-CoV-2.
CONCLUSIONS
Maternal mortality was associated with comorbidities such as obesity, hypertensive diseases of pregnancy, asthma and others, for both infections. In COVID-19 disease mortality was associated with higher maternal age (>30 years), which was not evident in H1N1 disease. H1N1 disease was associated with better outcomes when antiviral agent (oseltamivir) was administered within the first 48 hours of symptoms, while for COVID-19 there were no specific antiviral agents to be administered. The average days of mechanical ventilation were significantly different between the groups, since in the COVID-19 group they were significantly fewer. ECMO seemed to have better results in pregnant patients with COVID-19. Finally, both infections were related with preterm delivery, higher rate of cesarean section and neonatal admission to the NICU; however, neonatal mortality was slightly lower in the COVID-19 group.