INTRODUCTION
Mucormycosis is an aggressive, opportunistic fungal infection. It is originated from microorganisms that are part of the Zygomycete family, the order Mucorales and comprises several genera1. Immunocompromised patients (patients with hematological malignancies, transplant recipients, HIV, autoimmune diseases, immunosuppressive therapy) and patients with metabolic disorders (patients with uncontrolled diabetes mellitus, ketoacidosis) are mainly affected2. There have been no reported cases of transmission between humans. The primary way of getting infected is via inhalation of conidia, with other ways including ingestion and traumatic inoculation. The inflammation spreads through tissue or hematogenously, entering blood vessels and causing tissue infarction, necrosis, and thrombosis3.
Rhinocerebral mucormycosis is the predominant type of mucormycosis, followed by pulmonary, cutaneous, diffused, gastrointestinal and other organ infections4.
Only a small number of pulmonary mucormycosis cases have been documented in the literature (Table 1). The majority of these cases involves localized rather than invasive infection and is mostly related to underlying immunosuppression in a solid organ transplant setting4. Throughout the COVID-19 pandemic, a rise in cases of mycoses was reported – it was termed COVID-19-associated mucormycosis (CAM). A systematic review and meta-analysis conducted by Pal et al.5 in 2021 examined 99 patients with CAM, revealing that 10 of them had pulmonary mucormycosis. The main comorbidity of most patients in this meta-analysis was diabetes mellitus. Rai6, in a systematic review, reports 9 cases of pulmonary mucormycosis in people with severe COVID-19 disease with the main comorbidities being diabetes mellitus and hematologic malignancy.
Table 1
Most common mycoses of the lung
CASE PRESENTATION
An elderly male patient, 82 years old, visited the outpatient clinic with a past medical record of hypertension, type 2 diabetes mellitus under insulin treatment (HB 1Ac 7.2), end-stage chronic kidney disease (CrCl: 15 mL/min), and acute promyelocytic leukemia in remission. While hospitalized for maintenance therapy for acute promyelocytic leukemia (arsenic trioxide), he developed symptoms of a lower respiratory tract infection with nonproductive cough, dyspnea accompanied with fatigue and anorexia. During the clinical evaluation, the patient was found to be afebrile and hemodynamically stable. Upon auscultation, the patient presented with respiratory wheezing and presence of abundant crackles in the left hemithorax, mainly in the mid clavicular line. Blood gas exchange to atmospheric air (ABGs: FiO2=21%; pH=7.44; pCO2=28 mmHg; pO2=8228 mmHg) did not reveal hypoxemia. The electrocardiogram was in sinus rhythm, with right bundle branch block, which was preexisting. A chest X-ray was performed revealing opacity in the upper lobe of the left lung and a mild pleural effusion on the left. A PCR-test for COVID-19 was performed, with negative result. The laboratory tests demonstrated a rise in C-reactive protein (CRP): 18 (upper limit is 0.5 mg/dL) without leukocytosis and without neutropenia (WBCs: 5900/μL, Neu: 5600/μL). The remaining abnormal parameters in the complete blood test (anemia, Hb: 8.9 g/100 cc and thrombocytopenia, PLTs: 50000/μL) were influenced by the patient’s underlying hematological disease.
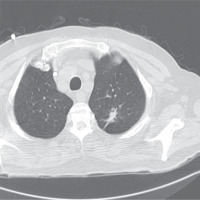
Further imaging was performed with a chest computed tomography, which revealed a solid pulmonary nodule in the apico-posterior part of the upper lobe of the left lung surrounding a bronchus with solid content, measuring 2.68 × 1.5 cm (Figure 1).
Figure 1
Pulmonary nodule in the apico-posterior part of the upper lobe of the left lung that surrounds a bronchus, measuring 2.68 × 1.5 cm
The patient received empiric antibiotic treatment with meropenem and linezolid to treat lower respiratory infection until pathogen isolation, as well as antifungal treatment with isavuconazole. The rest of the microbiological control testing included routine sputum cultures that identified staphylococcus of the normal flora and 3 samples of sputum cultures for B-Koch, which were negative. Also negative was the sputum cytology. Three days after the initiation of antifungal and antibiotic treatment bronchoscopy was performed with a flexible bronchoscope to obtain bronchoalveolar lavage (BAL). BAL was used to perform PCR for B-Koch, PCR for aspergillus and aspergillus antigen which were not detected. Furthermore, the serum galactomannan antigen test was tested, which was also negative. After the examinations, the differential diagnosis included fungal infection, adenocarcinoma of the lung and finally, less possibly, focal cryptogenic organizing pneumonia (COP) (Table 2).
Table 2
Differential diagnosis of pulmonary mucormycosis
| Tuberculosis |
| Nocardia |
| Aspergillus and other mycoses |
| Adenocarcinoma of the lung |
| Cryptogenic organizing pneumonia |
| Pulmonary embolism |
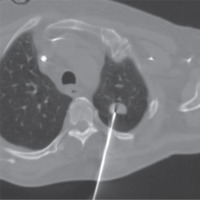
For the purpose of diagnosing the patient, a CT-guided biopsy was performed, initially uncomplicated (Figure 2). The result of the histological examination that was formulated a week later revealed a granulomatous necrotic inflammation of the lung with morphological characteristics of mucormycotic mycosis.
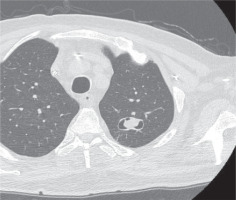
No surgical treatment was performed on the patient due to his severe pre-existing comorbidities. Anti-fungal therapy including a combination of liposomal amphotericin B and isavuconazole was offered, which the patient received for a total of nine weeks. To monitor the patient’s response to the treatment, three weeks after the start of the combined antifungal treatment, a chest CT scan was performed, which showed a reduction of the nodule and 6 weeks later in the repeated imaging, a further reduction of it was shown (Figure 3).
Figure 3
Three weeks after the beginning of the combined treatment, the pulmonary nodule is depicted in the apico-posterior part of the upper lobe of the left lung, comparatively reduced
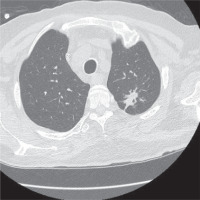
After receiving the combination anti-fungal treatment for a total of nine weeks, the patient was discharged from the hospital with instructions to take per os posaconazole, which he received for four weeks. Four weeks after his discharge, the patient underwent a new imaging test with chest CT, which, from the depiction of the lung parenchyma recognized ulcerative lesions in the apico-posterior part of the left upper lobe, with the pre-existing lesions nearly non-existent (Figure 4). The patient at the given time of the re-examination was clearly asymptomatic, with excellent tolerance to the medication. His quality of life improved drastically and he was able to return to his normal everyday activities.
DISCUSSION
Patients with hematologic malignancies, those who have undergone stem cell transplants and recipients of organ transplants are more susceptible to pulmonary mucormycosis7. In the last decade, there has been a notable rise in the incidence of mucormycosis, making it the second most important invasive fungal infection after aspergillosis in immunocompromised individuals8,9. Pulmonary mucormycosis usually appears with classic symptomatology of a lower respiratory tract infection sometimes combined with hemoptysis7. Diagnosing pulmonary mucormycosis is challenging due to the requirement of identification of the microorganisms in the tissue, with histopathology and culture confirmation needed10. Imaging tests may show a variety of findings such as masses, cavities, multiple nodules, focal consolidation, and pleural effusions. In a retrospective study, Chamilos et al.11 propose that the identification of a pleural effusion or of more than 10 nodules can assist in distinguishing between mucormycosis and aspergillosis in immunocompromised individuals. The halo sign and reversed halo sign are most commonly linked to invasive pulmonary aspergillosis and pulmonary mucormycosis, respectively12.
Once the diagnosis is determined, aggressive surgical debridement of the affected tissues should be considered as it has been linked to improved prognosis and survival outcomes13. Nevertheless, if there is extensive organ involvement and/or severe thrombocytopenia, surgery is not an option, and the patient will receive antifungal treatment instead. Pulmonary mycoses necessitate a combination of surgical intervention and antifungal therapy for treatment. Liposomal amphotericin B (intravenous) is the preferred drug for first-line treatment14. Upon clinical improvement, transition to stepwise de-escalation is done with the triazoles isavuconazole or posaconazole15,16. The latter are also utilized as a last resort therapy in individuals who do not tolerate amphotericin B or have a reason that prevents its use. Although liposomal amphotericin B is the first-line drug for invasive mucormycosis, there are several studies supporting that combination antifungal treatment has shown therapeutic benefit17,18. Despite the fact that the reported case received a treatment of liposomal amphotericin B and isavuconazole together, there is no evidence to support the advantages of combining antifungal treatments, and it is not suggested in standard treatment protocols. Patients with pulmonary mucormycosis have an average mortality rate of 50% to 70%, with a mortality rate of over 90% in mechanically ventilated patients in ICUs10,19.
CONCLUSION
With the rising occurrence of mucormycosis in the last two decades, it is important to consider mucormycosis as a potential diagnosis in immunocompromised patients. Prompt initiation of treatment and management of the underlying disease are crucial in preventing disease progression, enhancing the likelihood of cure.