INTRODUCTION
SARS-CoV-2, a new virus, first appeared in China in the city of Wuhan in December 2019. Since that day, this virus has expanded rapidly becoming a global pandemic, prompting the scientific community to mobilize to study this new disease1.
Initially, the respiratory symptoms associated with COVID-19 were considered to be of viral pneumonia. Subsequently, it has been shown that SARS-CoV-2 affects not only the respiratory system but also several other systems including the central nervous system (CNS). Thus, more and more neurological signs associated with SARS-CoV-2 have been reported1.
In addition, the SARS-CoV-1 virus has known neuroinvasive potential, and SARS-CoV-1 and SARS-CoV-2 have been shown to be approximately 80% similar. Therefore, it is possible to expect a neuro-invasive potential for SARS-CoV-2 as well2.
This possible neurological damage can either be due to the presence of SARS-CoV-2 in the CNS or to an inflammatory reaction without the presence of the virus in the CNS3.
Here, we report the case of a patient treated for COVID-19 who was diagnosed with cerebellar syndrome during hospitalization.
CASE PRESENTATION
The case was that of a 23-year-old man who presented to the emergency department with an acute, febrile, dry cough. The patient did not report any history of chronic alcoholism and no history of similar symptoms previously. The patient was not under any medication. A week prior to admission, he manifested a dry acute cough with dyspnea accompanied by loss of taste and smell, all progressing against a background of unstated fever. On the day following admission, vertigo and asthenia were added to the clinical signs. The vertigo was not accompanied by nausea, vomiting, or hearing loss.
The patient arrived at the hospital in a conscious state, well oriented in time and space, with: a temperature of 36.9℃, a blood pressure of 107/85 mmHg, a heart rate of 88 b/m, a respiratory rate of 21 breaths/min, and a pulsed oxygen saturation of 83% in ambient air and 97% under 4 L/min through oxygen cannula.
Neurological examination showed the following signs: normal cranial nerves examination, the patient was holding the Barré and not holding the mingazzini, normal muscle strength, no sensation disorders, normal osteotendinous reflexes, difficult standing position, cerebellar walking with widening of the sustentation polygon, coordination disorders with dysmetria which worsened when the eyes were closed. The remainder of the physical examination showed normal results.
The initial biological assessment showed: white blood cells at 5800/mm3 with lymphocytes at 1200/mm3 and neutrophils at 4200/mm3, hemoglobin at 15 g/dL, platelets at 131000/mm3, C reactive protein (CRP) at 86 mg/L, prothrombin level at 98%, activated partial thromboplastin time at 25 s, fibrinogen at 3.29 g/L, d-dimer at 10000 ug/L, lactate dehydrogenase at 530 IU/L, ferritin at 984.45 ug/L, normal transaminases, and levels of urea and normal creatinine.
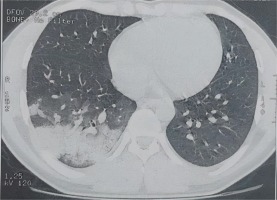
Chest computed tomography (CT) showed diffuse and peripheral bilateral ground glass opacities classified as CORADS 4 with pulmonary condensation with a small abundance of right pleural effusion (Figure 1).
Figure 1
Thoracic CT transverse section: diffuse and peripheral bilateral frosted glass opacities classified CORADS 4 with pulmonary condensation
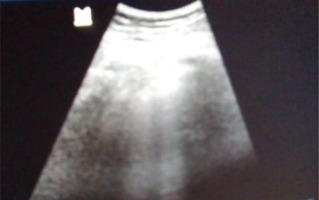
Chest ultrasound exploration showed a condensation syndrome with coalescing B lines (Figure 2).
Figure 2
Thoracic ultrasound in longitudinal section by deep probe of 3.5 Mega-Hertz showing condensation syndrome with coalescing B lines
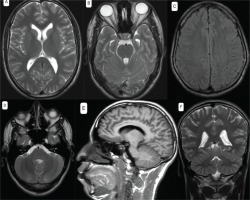
The nasopharyngeal SARS-CoV-2 polymerase chain reaction (PCR) was positive. Brain CT scan showed bilateral posterior parietal and right central oval subcortical hypodense lesions not enhanced after contrast.
Brain exploration by magnetic resonance imaging (MRI) was also performed and presented a normal result (Figure 3). Lumbar puncture was performed with a clear liquid and showed: leukocytes <3/mm3, red blood cells at 160 E/mm3, CSF protein at 0.23 g/L, glycorrhachia at 0.73 g/L and a negative culture.
Cerebrospinal fluid (CSF) PCR test for SARS-CoV-2 was negative.
The list below shows the therapeutic protocol followed by the patient:
Hydroxy chloroquine 200 mg, 3 times daily for 7 days oral administration;
Azithromycin 500 mg/day on the first day and 250 mg/day from the second to the seventh day oral administration; and
Methylprednisolone 120 mg/8 hours for 10 days in intravenous administration.
The evolution was marked by a gradual regression of neurological signs, especially after several sessions of motor physiotherapy with the need for anti-anxiety and antidepressant treatment after psychiatric evaluation.
Samples and diagnostic tests
Nasopharyngeal samples were collected via swabbing and placed in a single sterile tube. Two milliliters of CSF were poured into another sterile tube.
In the molecular biology laboratory, the study was carried out first by extracting the messenger RNA (the kit used is the Gene Finder mixed kit), then an addition of the extraction plates with a mixed plate was carried out before putting the plate in a thermocycler. The results are obtained in the form of curves containing the envelope E genes, the N gene, and the RNA polymerase (RdRp) gene with a positivity threshold of 40.
DISCUSSION
Neurological involvement is possible for the SARS-CoV-1 virus, which has been shown to be neuroinvasive in several studies. Therefore, SARS-CoV-2 can also induce neurological signs.
Angiotensin-converting enzyme-2 (ACE2) receptors are suspected of being responsible for the transport of the SARS-CoV-2 virus. They are present in the respiratory and vascular epithelium, which may explain the observed manifestations, but they are also present at the neuronal level and in the cerebral vascular endothelium which the virus can use to enter the CNS4.
This neurological damage caused by SARS-CoV-2 may be due to hypoxia, direct viral invasion of the CNS (either by hematogenous dissemination or by retrograde neuronal dissemination from the olfactory mucosa), or to a generalized inflammatory reaction (cytokine storm) following viral infection such as elevation of ferritinemia, CRP and interleukin-6 leading to a ‘state of hyper-inflammation’ which may be due to a possible deregulation of the monocyte macrophage system3,5,6.
Respiratory worsening in some patients may be due to involvement of the cardio-respiratory centers in the CNS5.
A first case of acute cerebellitis was reported in a 47-year-old man with a cerebellar syndrome and a PCR for SARS-CoV-2 positive at the nasopharynx and CSF (the particularity of this case is the positivity of SARS-CoV-2 in the CSF) which suggests that the direct involvement and the presence of the virus in the CNS is the most likely pathological mechanism7.
Another study of four cases, treated for various neurological signs that appeared during hospitalization for SARS-CoV-2 infection, showed the absence of SARS-CoV-2 in CSF after a negative SARS-CoV-2 PCR in all of these patients, suggesting that the generalized inflammatory reaction is the most likely pathological mechanism8.
In our patient, a cerebellar syndrome with a positive SARS-CoV-2 PCR at the nasopharynx and negative in the CSF with an increase in CRP and ferritinemia, and given the absence of any history of similar symptoms or medications or metabolic diseases and the exclusion of vascular causes by the brain CT, all of this suggests that the mechanism that may explain this neurological impairment is the inflammatory reaction.
The actual presence of the virus in the CNS cannot be ruled out when the CSF test for SARS-CoV-2 is negative. It is therefore possible to question the sensitivity of the SARS-CoV-2 PCR in the CSF which is not well known until now to our knowledge, hence the interest of integrating the other diagnostic elements to establish the diagnosis of neurological and meningeal involvement.
Several more studies are needed on the neurological involvement associated with SARS-CoV-2 to better understand the mechanism involved and to study the sensitivity of SARS-CoV-2 PCR in CSF.