INTRODUCTION
Sarcoidosis is a multi-systemic disease of unknown etiology characterized by non-caseating granulomas at the sites of disease1. A rare manifestation of this disease is miliary sarcoidosis where numerous miliary-like nodules are randomly distributed throughout the lungs. In this article, we report two cases of miliary sarcoidosis, diagnosed and treated successfully in our department.
CASE PRESENTATION
Case 1
Α 54-year-old female patient was referred to our hospital due to low oxygen saturation. She reported progressively worsening dyspnea and non-productive cough in the last 2 months. Her medical history was significant for peptic ulcer, dyslipidemia, systemic hypertension and morbid obesity. There was no history of allergy or occupational exposure. On admission, the patient presented with tachycardia (110 beats/min) and oxygen saturation of 90% on room air. Lung auscultation revealed bilateral fine crackles, while the rest of the physical examination was unremarkable.
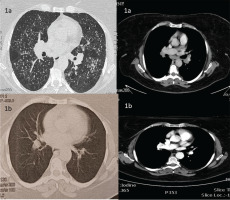
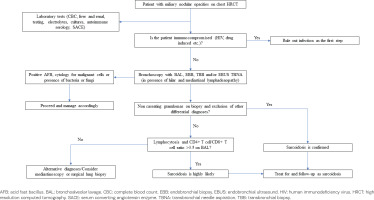
Chest X-ray revealed reticulonodular pattern and bilateral hilar enlargement. High resolution computed tomography (HRCT) of the chest showed numerous miliary-like nodules in both lungs along with hilar and mediastinal lymphadenopathy (Figure 1a). A focused diagnostic algorithm was performed on the basis of miliary nodular lung pattern (Figure 2). Tuberculin skin test was negative and laboratory testing was normal. Autoimmune serology and levels of serum angiotensin converting enzyme (ACE) were normal. Pulmonary function testing revealed mild restriction with normal diffusion capacity of carbon monoxide.
Bronchoscopy showed no evidence of endobronchial inflammation or lesion. Bronchoalveolar lavage (BAL) consisted of 79% macrophages and 21% lymphocytes, of which 78% CD4+ T and 2% CD8+ T [(CD4+ T)/(CD8+ T) >3.5]. Microbiological cultures were negative for bacteria, fungi and mycobacteria, while cytology examination of BAL was negative for malignant cells. Unfortunately, we did not perform transbronchial biopsies (TBB), due to the patient’s worsening oxygenation during the procedure.
Based on these results, a diagnosis of pulmonary sarcoidosis was set. The patient received 40 mg prednisolone for 6 months, and at 12 months, without treatment, the patient was asymptomatic with an oxygen saturation of 99% on room air and had complete remission of HRCT findings (Figure 1b).
Case 2
A 35-year-old female patient referred to our hospital due to chronic non-productive cough and micronodular lesions in both lungs evident on chest X-ray. She had no previous medical history and absence of occupational exposure or known allergy, with the exception of active tobacco smoking (15 packs per year). On physical examination, she was afebrile, with an oxygen saturation of 98% on room air. Lung auscultation revealed mild inspiratory fine crackles in both lower lung zones, while the rest of the findings were normal.
HRCT of the chest revealed bilateral multiple micronodules and tree-in-bud pattern in both lungs, mainly in the upper, middle lobe and lingula, accompanied by enlarged lymph nodes in the mediastinum and the pulmonary hila (Figure 3a). The same diagnostic algorithm was followed (Figure 2). Laboratory and pulmonary function tests were normal. Fiberoptic bronchoscopy had normal macroscopic findings with BAL consisting of 92% macrophages and 8% lymphocytes and normal (CD4+ T)/(CD8+ T) of 1.8. Microbiology and cytology examination of BAL were normal. There was no evidence of any other organ involvement.
Figure 3
HRCT of the thorax of the second case before (a) and after treatment with glucocorticoids (b)
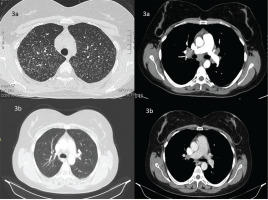
Due to the inconclusive findings, we proceeded with mediastinoscopy. Histological examination of mediastinal lymph nodes indicated non-caseating granulomatous inflammation with negative Ziehl–Neelsen staining and culture for mycobacterium tuberculosis and fungi on the histological specimen. Sarcoidosis was diagnosed and the patient received 40 mg prednisone with a gradual tapering for 6 months until it was stopped. At 12 months, the patient was asymptomatic (without treatment) with an oxygen saturation of 98% on room air and improved chest HRCT findings (Figure 3b).
DISCUSSION
Sarcoidosis is a chronic condition of unknown origin, characterized by the presence of non-caseating granulomas in the involved organs, usually in the lungs2. Typically, sarcoidosis manifests with bilateral hilar adenopathy, pulmonary reticular opacities and skin lesions; however, other organs may be also affected1. The average age of onset is between 40 and 55 years, and is more often found in women (45–60% of cases) than men2. Thoracic manifestations of sarcoidosis are divided into typical and non-typical. Typical manifestations include hilar lymphadenopathy, micronodules with a perilymphatic distribution, and bilateral perihilar opacities, while as non-typical are considered the following: alveolar and miliary opacities, honeycomb-like cysts, mosaic pattern, and pleural disease3.
Miliary sarcoidosis is a non-typical and rare presentation of sarcoidosis representing <1% of the reported cases4. The relation of micronodules to the secondary pulmonary lobule could aid in the proper diagnosis. They could be centrilobular, perilymphatic or randomly distributed5. The miliary pattern describes the appearance of innumerable micronodules <3 mm in diameter with a random distribution and implies a hematogenous dissemination of the disease5. In miliary sarcoidosis these nodules have a random pattern without an obvious perilymphatic distribution and may mimic a typical of miliary tuberculosis.
Although, a number of cases with miliary sarcoidosis have been described in the literature4, some cases tend to have a ‘pseudo-miliary’ pattern. A recent systematic review of the published cases reported that some patients with miliary sarcoidosis presented also subtle micronodules with a perilymphatic distribution on HRCT4. The most common diseases with a miliary pattern refer to infections (mainly tuberculosis and fungi; namely histoplasmosis, coccidioidomycosis, and hematogenous candida infection), pneumonoconiosis, and pulmonary metastases. Other causes like hypersensitivity pneumonitis, some bacterial infections (e.g. mycoplasma pneumoniae and hemophilus influenzae) and other rare diseases could present with pseudo-miliary pattern and should be searched for4.
The diagnostic approach is analogous to that of sarcoidosis2. Sarcoidosis is diagnosed on the basis of compatible clinical findings, supported by the presence of non-caseating granulomas in one or more tissue samples, and after excluding other causes of granulomatous disease6. Chest HRCT followed by bronchoscopy are crucial for the diagnosis, and they will narrow the differential diagnoses. The triad of compatible clinical features, BAL lymphocytosis, and increased (CD4+ T cell)/(CD8+ T) cell ratio (>3.5) may support the diagnosis of sarcoidosis2,7, as in our first patient who did not tolerate the bronchoscopy for TBB sampling. Nevertheless, a tissue sampling should be made, either TBB or endobronchial biopsy in case of endobronchial lesion, in order to confirm the presence of granulomatous inflammation. In case of enlarged hilar and/or mediastinal lymph nodes, endobronchial ultrasound transbronchial needle aspiration (EBUS-TBNA) – with or without biopsy – of the affected lymph nodes should be the first option when the institution has access to it. At that time, we did not have access to EBUS-TBNA and thus we supported our diagnosis with the clinical findings and the compatible BAL results. In selective cases, a mediastinoscopy or surgical lung biopsy may be required to confirm the diagnosis, as in our second case.
Glucocorticoids remain the mainstay for patients with miliary sarcoidosis, while immunosuppressants are given to refractory cases4,8. Oral glucocorticoids are usually given at doses of 20 to 40 mg prednisone for 2 to 6 weeks and then tapered carefully for 6–12 months, depending on disease response8. Of note, lower protective doses of prednisone (10 mg daily) may be also effective for long-term and insidiously progressive disease7,9. Moreover, follow-up includes radiographic examination and evaluation of pulmonary function testing, while emphasis should be placed on potential serious complications, such as cardiac and neurologic involvement1.
In both patients we followed the above-mentioned investigation procedure (Figure 2). Alternative diagnoses were excluded based on the HRCT and bronchoscopy findings (Case 1) and the histopathologic results (Case 2) in line with history and clinical examination. The response to glucocorticoid treatment and the follow-up confirmed our diagnosis in these cases. A close monitoring of them is ongoing.
CONCLUSION
Miliary sarcoidosis is a rare pulmonary manifestation of sarcoidosis where numerous micronodules are randomly scattered throughout the lungs. Clinicians should have a high index of suspicion and follow a structured diagnostic approach to diagnose miliary sarcoidosis and exclude other causes with similar presentation, mainly tuberculosis. Treatment of miliary sarcoidosis requires glucocorticoids at doses similar to the typical sarcoidosis, while a close monitoring and follow-up is of crucial importance.