INTRODUCTION
Tuberculosis (TB) remains one of the major causes of morbidity and mortality in the world, since, according to WHO, TB was the leading cause of death from a single infectious agent, ranking above HIV/AIDS until the coronavirus (COVID-19) pandemic1. The progress noted in TB epidemiology at a global level was severely affected by the pandemic, which had a damaging impact on TB diagnosis, burden, and number of deaths. The most evident effect was a drop in the reported number of new TB notifications. From a peak of 7.1 million in 2019, it fell to 5.8 million in 2020 (-18%), which is back to the level of 2012. In 2021, there was a partial recovery to 6.4 million (the level of 2016–2017), according to WHO data, but, in total, the progress made in the years up until 2020 has been reversed, with global TB targets being off track much more ever since2.
According to WHO’s TB profile for Greece, 195 new cases were registered in 2021 (incidence rate: 4.1 per 100000 population)3. Underreporting is a serious problem for TB surveillance in Greece, while there is no TB treatment outcome registry. Up until 2011, Greek data were not provided to WHO, therefore Greece was not included in the study on TB treatment outcome in the European Union and the European Economic Area4. Furthermore, Greece is one of a few countries where TB treatment outcome is neither reported nor registered, according to the e-CDC (Bulgaria, France, Greece, Italy, Latvia, Poland in 2020)5. Recording treatment outcome, assessment of program performance, and the recording epidemiological trends provide the basis for programmatic and policy development; therefore, they are essential for identifying and assessing problems in treatment algorithms, as well as TB control in general, worldwide6.
The present study aims to assess the outcome of tuberculosis treatment at the Department of Pulmonary Medicine, AUTH, at ‘G. Papanikolaou’ General Hospital of Thessaloniki, and to identify the factors potentially associated with a negative outcome. Specifically, TB treatment outcome was studied based on the time treatment was initiated (before or during the COVID-19 pandemic) and on the patients’ country of origin, as about half of the study population is not Greek. This comes in accordance with Greek Public Health Organization data7.
METHODS
This is a retrospective study of patients with TB disease, registered at the Department of Pulmonary Medicine, Aristotle University of Thessaloniki, between 1 January 2018 and 31 December 2021. The department functions as a TB reference center for the regions of West and Central Macedonia. TB outcome definitions suggested by WHO were used1.
TB outcome definitions
Cured
Patients with pulmonary TB with bacteriologically confirmed TB at the beginning of treatment who completed treatment, as recommended by the national policy, with evidence of bacteriological response – conversion with at least two consecutive cultures – and no evidence of failure.
Treatment completed
Patients who completed treatment, as recommended by the national policy, whose outcome does not meet the definition for cure or treatment failure.
Treatment failed
Patients whose treatment regimen needed to be terminated or permanently changed to a new regimen or treatment strategy.
Died
Patients who died before starting treatment or during the course of treatment.
Lost to follow-up
Patients who did not start treatment or whose treatment was interrupted for two consecutive months or more.
Not evaluated
Patients for whom no treatment outcome was assigned (e.g. patients transferred-out to another treatment unit).
In this study, TB treatment outcomes were also categorized in three different groups: positive, negative, or death. Positive outcome was defined as cure and completion of treatment while negative outcome was defined as loss to follow-up, failure, and no evaluation. Death was assessed separately. Although outcome definitions are the same for TB due to sensitive and resistant strains, patients with rifampicin-resistant or multidrug-resistant TB were excluded from the study since treatment for MDR-TB is longer and outcomes could not be assessed for the year 20218.
Apart from outcome, the following parameters were recorded for each patient: age, gender, country of origin, co-morbidities, and anatomical site of TB infection. Regarding co-morbidities, the Charlson index9 was used as a tool to assess long-term mortality. Moreover, the diagnostic method (nucleic acid amplification test [NAATs] – Xpert® MTB/RIF Assay, Cepheid, California, US, acid-fast bacilli [AFB] smear, culture, histological), resistance profile, time to negative smear/cultures and duration of treatment, as well as outcome and possible adverse effects, were recorded.
Patients were divided into two groups based on time of diagnosis. The first group consisted of patients who started treatment before the beginning of COVID-19 pandemic, i.e. January 2018 – February 2020, and the second one during COVID-19, i.e. March 2020 – December 2021. Patients were also divided according to country of origin, i.e. Greece, born in Europe (apart from Greece), and born elsewhere. After March 2020, TB patients were tested for SARS-CoV-2 infection as part of the initial differential diagnosis. At follow-up, TB patients were tested only if an indication of viral infection was present. TB outcomes as well as demographic and microbiological parameters were compared between groups.
RESULTS
In total, 102 patients, 15 (14.7%) women and 87 (85.3%) men, with mean age 44.8 ± 21.9 years (range: 17–87) were included in the study. The characteristics of participants are presented in Table 1. Fifty-seven patients (55.9%) were of foreign origin: Pakistan (19), Somalia (6), Georgia (6), Afghanistan (4), Albania (3), Syria (3), Guinea (2), Iraq (2), Ukraine (2), Congo (2), Armenia (1), Iran (1), Cameroon (1), Mali (1), Bangladesh (1), Bali-Indonesia (1), Romania (1) and Senegal (1). Forty-five patients were born in Greece, representing 44.1% of all the sample. Pulmonary TB was diagnosed in 80 (78.4%) patients, with 12 having both pulmonary and extra-pulmonary disease, and two presenting with miliary TB. Extra-pulmonary TB alone was diagnosed in the remaining 22 patients (21.6%). Regarding the location of extra-pulmonary TB, most patients presented with TB lymphadenitis (9 cases, 40.9%), but involvement of pleura, kidney and testicle were also registered. Previous anti-TB treatment was reported in four of the cases, and HIV co-infection in one. Fifty-eight patients (56.9%) lived with comorbidities, including HCV or HBV infection, active malignancy or history of cancer, cardiovascular disease, diabetes, renal disease, autoimmune diseases, and inflammatory bowel disease, and four of them were also illicit drug users. None of the patients suffered from COVID-19 during their hospitalization.
Table 1
Patients’ characteristics
Patients of Greek origin were significantly older with mean age 62.3 ± 15.5 versus 50.0 ± 16.9 in other Europeans, and 26.1 ± 10.5 in non-Europeans (p<0.001). They also had a higher rate of coexisting comorbidities, as indicated by a Charlson comorbidity index of 3.4 ± 2.7 vs 1.6 ± 1.8 in other Europeans, and 0.2 ± 0.7 in non-Europeans (p<0.001).
Out of 99 patients for whom the method of diagnosis was registered, microbiological confirmation was achieved in 86 patients (84.3%), and histological in 13 (12.7%). Patients with histological diagnosis of TB suffered mainly from extra-pulmonary TB, or from both pulmonary and extra-pulmonary TB. Specifically, 9 patients had extra-pulmonary TB (3 patients had lymphadenitis, 4 patients had pleurisy, 1 patient had peritonitis, and 1 patient testicular TB). Of the other 4 patients, one had both pulmonary and extra-pulmonary TB (TB pleurisy), and was diagnosed with pleural biopsy, while the other 3 had only pulmonary TB, and were diagnosed with lung biopsy. In these cases, radiological findings were mainly pulmonary nodules. TB was diagnosed clinically in 3 cases (3%), all of them being before the pandemic outbreak.
Regarding outcome, out of 102 patients, 60 had a positive outcome, accounting for 58.8% of cases, while 35 (34.3%) had a negative outcome. Seven patients died, all of whom had comorbidities (heart conditions, underlying pulmonary disease, or cancer). Three of these deaths were attributed to causes other than tuberculosis disease, a car accident in one case, and metastatic lung cancer in the other two. Four of the patients who died had experienced side effects from TB treatment (such as drug-induced hepatitis, neuropathy, and psychosis). Out of these cases, only one (drug-induced hepatitis and hepatic failure in a woman aged 84 years with pulmonary fibrosis, diabetes, and hypertension) was associated with death, shortly after the beginning of treatment. In the three remaining patients with side effects, death took place after the side effects had been addressed and treatment had been accordingly modified. Three of the patients who died were diagnosed after the COVID-19 outbreak, but none of them tested positive for the virus.
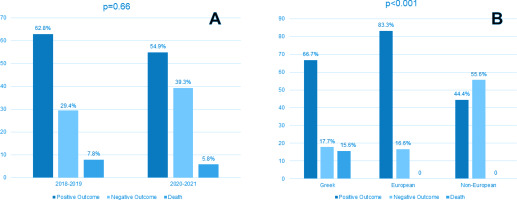
Treatment outcome according to year of diagnosis (before and during the pandemic) is presented in Table 2. A statistically significant difference in treatment outcome between the pre-COVID-19 and the COVID-19 period was observed (p=0.02), with 43.1% vs 21.6% achieving cure before and during the pandemic, respectively. The percentage of patients who were lost to follow-up increased from 2% before COVID-19 to 15.7% during COVID-19. However, when outcomes were grouped (positive, negative, death) no difference between the periods was observed (Figure 1 A).
Table 2
Treatment outcome according to year of diagnosis
| Outcome* | Pre-COVID-19 (N=51) n (%) | During COVID-19 (N=51) n (%) | Total (N=102) n (%) |
|---|---|---|---|
| Cured | 22 (43.1) | 11 (21.6) | 33 (32.4) |
| Treatment completed | 10 (19.6) | 17 (33.3) | 27 (26.5) |
| Treatment failed | 0 (0.0) | 1 (2.0) | 1 (1.0) |
| Lost to follow-up | 1 (2.0) | 8 (15.7) | 9 (8.8) |
| Not evaluated | 14 (27.5) | 11 (21.6) | 25 (24.5) |
| Died | 4 (7.8) | 3 (5.9) | 7 (6.9) |
As shown in Table 3 and Figure 1 B, treatment outcome differed significantly according to origin, with positive outcome observed in 66.7%, 83.3% and 44.4% in Greeks, other Europeans, and non-Europeans, respectively (p<0.001). All patients who died were Greek. Treatment outcome did not differ according to gender, site of infection, method of diagnosis or presence of resistance. Age differed significantly among patients with positive outcome, negative outcome, or death (47.9 ± 20.0, 34.9 ± 19.9, and 68.0 ± 24.0, respectively, p<0.001). In addition, the Charlson co-morbidity index was significantly higher in patients who died (1.6 ± 1.7, 1.2 ± 2.2, and 6.9 ± 3.6, for patients with positive outcome, negative outcome, and death, respectively, p<0.001).
Table 3
Treatment outcome according to country of origin
| Outcome* | Greece (N=45) n (%) | Other European (N=12) n (%) | Non-European (N=45) n (%) |
|---|---|---|---|
| Cured | 18 (40.0) | 5 (41.7) | 10 (22.2) |
| Treatment completed | 12 (26.7) | 5 (41.7) | 10 (22.2) |
| Treatment failed | 1 (2.2) | 0 (0.0) | 0 (0.0) |
| Lost to follow-up | 2 (4.4) | 0 (0.0) | 7 (15.6) |
| Not evaluated | 5 (11.1) | 2 (16.7) | 18 (40.0) |
| Died | 7 (15.6) | 0 (0.0) | 0 (0.0) |
DISCUSSION
The main results of the present study are: 1) outcome groups (positive, negative or death) in the pandemic era did not differ from the pre-COVID-19 period, however, when all WHO groups were analyzed, a significant difference was observed; and 2) outcome was affected by country of origin, with patients of non-European origin presenting with the higher percentage of negative outcomes, and Greeks accounting for all the deaths.
The positive outcome rate post-pandemic (54.9%) had no statistically significant decrease in comparison to those of the period before (62.8%). At the same time negative outcome also appeared to be rather unaffected by the pandemic (39.2% vs 29.4%). In our view this fact reflects stability in TB management, which was mainly the result of the effort and engagement of staff members, some of whom were involved exclusively in TB. Indeed, one of the three doctors of the outpatient clinic, and the specialized TB nurse, did not participate in the treatment of COVID-19 patients. These members of staff were a crucial part of the operational efficiency of the outpatient clinic. Although not confirmed in our center, TB is considered to be a risk factor related to worse COVID-19 prognosis10. Tuberculosis and SARS-CoV-2 co-infection is not well-studied worldwide, and more data are needed to better understand them when they occur together.
Lockdown has favored the increased use of telemedicine, a means of health service that can easily be provided by TB programs. In TB centers surveyed in Australia, Russia, India, and the United Kingdom, telehealth service use increased in the first 4 months of 2020 according to the CDC11. An increased use of telehealth during the COVID-19 pandemic was observed in some TB centers worldwide12. As many of our patients come from remote parts of Northern Greece, telehealth has always been part of our clinic’s work, and staff were well-familiar with it, and were thus able to immediately incorporate it into everyday routine for the majority of patients. For example, laboratory exams were remotely performed and sent to the clinic’s email. After that, scheduled telephone appointments were periodically held by doctors for stable patients, allowing them to have physical presence appointments every three months instead of every month. Telemedicine is probably the explanation for the low ‘cure’ but high ‘treatment completed’ rates post-COVID-19 versus the pre-pandemic era. This reflects that patients responding to treatment were managed from a distance without sputum results during the course of treatment. Sputum negativity is a perquisite for establishing ‘cure’ according to WHO definitions13.
A significant rise in patients lost to follow-up was noted (8 during COVID-19 versus only one before COVID-19), marking the effect of the pandemic on TB control. An increase in the proportion of cases who are lost to follow-up is a worldwide phenomenon. According to a study in Northern Italy performed in 2020, the rate of patients lost to follow-up escalated from 2.6% to 10.8% due to the COVID-19 outbreak, respectively14. Patients canceled or postponed follow-up examinations, because of fear of infection with COVID-19 when visiting healthcare environments, objection of family members, or feeling lack of necessity15. In addition, tuberculosis patients diagnosed during the COVID-19 pandemic showed more extended pulmonary forms16. Regarding our center, our hospital has been a reference center for COVID-19 since March 2020, therefore the fear of stigma and of contamination at visits was unavoidable. It is also located away from the city center, and, as a result, transportation has always been a major inconvenience, even before the pandemic outbreak.
Reasons for the reduction in TB diagnosis may include decreased attention to TB by healthcare systems, difficulties in accessing health services, lockdown measures, and fear of stigma and contagion. During the pandemic, a significant amount of TB patients reported difficulty in transportation, particularly the lack of available vehicles and/or the high cost of travel. Receiving treatment from directly observed treatment programs from clinics was also highlighted as a barrier due to fear of contracting an infection17.
Country of origin appears to be a major factor affecting outcome in our study, as patients from non-European countries showed the highest negative outcome rate. Lack of a support system (familial and/or social) was noted in most of the patients of non-European origin in our center, who are mainly immigrants and/or war refugees, and come to Greece unaccompanied by family members. Furthermore, such socially vulnerable groups are prone to other conditions associated with poor treatment outcomes, such as homelessness or illicit drug use, which, according to a Brazilian study by Chenciner et al.18, are the two main factors leading to unfavorable results of TB treatment. In contrast, patients from European countries usually migrate in family groups, which provide support for patients. The lack of state-organized infrastructure for migrants with TB (such as a patient-centered approach with directly observed therapy, provision of food and residence, and social support) can probably explain the high lost to follow-up rate in non-European patients. The impact of social protection programs on adults with TB has been analyzed in several studies, which have demonstrated that they are associated with improvement in treatment, cure rates, treatment adherence, service provision, poverty, and TB control19.
Another interesting finding of the present study was the number of deaths which accounted for 6.9% of patients. A systematic review of risk factors for death in adults during and after TB treatment reported that risk factors for death, in settings with high TB incidence and HIV prevalence, were co-infection with HIV, advanced immuno-compromised patients, smear-negative TB, and malnutrition. In regions of low TB incidence and HIV prevalence, like Greece, risk factors included non-infectious co-morbidities, sputum smear-positive TB, and alcohol and substance abuse20. Our study results come in accordance with these findings, as deaths occurred in Greeks, who were older, and with significantly more comorbidities in comparison to the other groups (although only 27.5% had sputum smear positivity). TB mortality is generally low in several studies, with most patients dying with comorbidities (malignancy, liver cirrhosis, etc.) and even because of them21. Migrants tend to be of younger age and previously healthy, therefore it comes as no surprise the zero deaths in this group.
Limitations
The main limitation of our study is that only one center, with a relatively small number of patients, is represented and therefore it is difficult to estimate whether our results can be extrapolated to the whole country. The circumstances especially regarding COVID-19 and the clinic’s operating conditions may vary in other centers. Therefore, possibly the results regarding the effect of the pandemic on TB outcomes may be different in other centers, where the degree of involvement for the care of COVID-19 patients, as opposed to TB patients, was different to ours. On the other hand, the impact of country of origin on TB outcomes shown in the present study probably reflects the situation in Greece in general, as the social protection status is the same for the whole country.
CONCLUSIONS
The results of this single-center study show that the positive outcome rate of TB patients was in the most part not severely affected by the COVID-19 pandemic. On the other hand, the country of origin of the patients was a determining factor of outcome, with non-Europeans presenting with the higher rate of negative outcome. Since the reasons for that are mainly socioeconomic, development of a national anti-TB program providing financial and social support, especially for the Middle East and African immigrants in Greece, would optimize TB treatment outcome and raise the positive outcome rate closer to the WHO global target. A multi-center study to assess all TB treatment outcome data from Greece would be of great importance to better understand Greek TB patients’ profile, omissions on screening and diagnostic evaluation, and treatment underachievement. The need for a national anti-TB program to be scheduled and implemented is crucial, not only for the improvement of treatment outcomes, but also to establish alignment with WHO global targets and WHO’s End TB Strategy.