INTRODUCTION
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has had a great impact on nursing homes1. Nursing homes, as facilities predominantly serving older persons in need of 24-hour health and care services, house a vulnerable, clinically complex group marked by physical and mental multimorbidity as well as diminished function in activities of daily living2-4. According to published data, the majority of nursing home residents are individuals aged >80 years, with most of them suffering from dementia or cognitive dysfunction, and each resident is likely to have three or more diagnoses for both mental and physical conditions3-5. Older individuals (≥65 years) with at least one underlying disease have a greater possibility of experiencing severe COVID-19 than their younger counterparts. Besides that, the risk of mortality from COVID-19 increases with age, with those aged ≥80 years being at the highest risk of death6-9.
The COVID-19 pandemic affected nursing homes particularly hard, with recurrent outbreaks and significant fatality rates10,11. Visitation limits, cohorting, and isolation have been proposed as preventive measures for viral transmission and outbreaks of COVID-19 in nursing homes. As a result, the additional stresses associated with such pandemic measures are likely to have had a significant detrimental impact on both the mental and physical well-being of many nursing home residents2.
Nursing home residents are especially vulnerable to infectious diseases in comparison with other individuals because they are characterized by both physical and mental multimorbidity, an impaired immune system, older age, low functioning in activities of daily living, increased frailty, high reliance on nursing staff, and cohabitation in grouped living facilities3,4,10. Atypical infection presentation, such as the absence of fever, delirium, and falls, is common among nursing home residents12-14. In this context, identifying infectious illnesses in the general population might be difficult12,13.
Several studies have evaluated the demographic and clinical characteristics, comorbidities, clinical presentations, and mortality of nursing home residents hospitalized due to COVID-19. However, most of them refer to the first pandemic waves, or they compare the delta with the omicron variant prevalence period15-17. Our study is one of a handful of studies to evaluate the characteristics, clinical manifestations, in-hospital mortality, and 90-day mortality of elderly (≥65 years) nursing home residents who were hospitalized due to COVID-19 pneumonia in a long period of the pandemic.
METHODS
Study design
The present study retrospectively analyzed data from elderly nursing home residents aged ≥65 years who were hospitalized with COVID-19 pneumonia in the Department of Infectious Diseases-COVID-19 Unit of Laiko General Hospital from 15 February 2021 to 31 December 2022. This research was conducted in accordance with the principles set forth in the Declaration of Helsinki and was approved by the Laiko General Hospital Institutional Review Board (protocol number 7950/08.06.2023).
The following criteria for inclusion were applied: 1) age ≥65 years; 2) nursing home residency; 3) polymerase chain reaction confirmation of COVID-19; and 4) severe or critical disease according to the clinical spectrum of SARS-CoV-2 infection [https://www.covid19treatmentguidelines.nih.gov/]. The exclusion criterion was the lack of data on 90-day survival post-diagnosis.
Data collection
Demographics, clinical manifestations, vaccination status against COVID-19, the Charlson comorbidity index (CCI), data from complete blood count, biochemical parameters and blood clotting parameters were recorded upon admission.
Statistical analysis
We employed the Kolmogorov-Smirnov test to evaluate the normal distribution of the parameters. Continuous variables with a normal distribution are presented as mean (standard deviation), while those with a non-normal distribution are presented as median (range). For the comparison of normally distributed continuous variables, we used the Student’s t-test, and for the non-normally distributed continuous variables we used the Mann Whitney U test. For the comparison of the categorical variables between the groups we used the chi-squared test and the Fischer’s exact test.
To identify predictive factors for the event(s) – defined as in-hospital mortality or mortality within 90 days – statistically significant variables were assessed using Cox proportional hazards univariable and multivariable regression analysis. The proportional hazards assumption was assessed and met for the Cox regression models. The discriminatory power of significant parameters was evaluated through the area under the receiver operating characteristic curve (ROC). Effect size is reported as hazard ratios (HRs) with 95% confidence intervals (CIs). The area under the curve (AUC) was calculated using the DeLong test. Values of p<0.05 were considered to suggest statistically significant differences. All statistical tests were two-tailed. Statistical analysis was conducted using IBM SPSS-Statistics version 26.0 (IBM Corp.).
RESULTS
During the study period, a total of 1398 elderly individuals were hospitalized in our unit due to COVID-19 pneumonia, of whom 90 (6.4%) patients (48 females, 53.3%) were nursing home residents. The mean age of the patients was 83.67 (SD=8.91) years, ranging from 65 to 101 years old. The median value of the CCI was 5, ranging from 2 to 10. During the study period, 48 patients (53.3%) were hospitalized when the alpha variant predominated, 14 patients (15.6%) were hospitalized during the delta variant predominance, and 28 patients (31.1%) were hospitalized during the omicron variant predominance.
The most common clinical symptom was dyspnea (66 patients, 73.3%). Regarding the vaccination status, only 32 (35.5%) patients were fully vaccinated (at least 3 doses, 28 had received only the mRNA vaccine, 2 patients had received one dose of Ad26.COV2. S COVID-19 followed by one dose of mRNA vaccine and 2 patients received 2 doses of the ChAdOx1 nCoV-19 vaccine followed by one dose of mRNA vaccine). Forty-six (51.1%) patients died during their hospitalization, while a total of 56 (62%) patients died during the first 90 days after diagnosis. None of the nursing home residents had received antiviral treatment before admission and none of them was treated with non-invasive ventilation or was intubated.
The analysis of the 90-day mortality data revealed significant differences in age and Charlson comorbidity index (CCI) between survivors and non-survivors. Non-survivors had a higher mean age (86.65 ± 7.21 years) compared to survivors (80.44 ± 8.97 years) (p=0.001), and a higher median CCI (5, range 4–10) compared to survivors (4.5, range 2–7) (p=0.001). Other parameters, including gender, variant type, dyspnea, cough, gastrointestinal symptoms, altered mental status, and vaccination status, did not show statistically significant differences in mortality rates. Specifically, the p-values for gender (0.829), variant type (0.133), dyspnea (0.088), cough (0.077), gastrointestinal symptoms (0.141), altered mental status (0.999), and vaccination status (0.999) indicated no significant association with 90-day mortality. These findings suggest that advanced age and higher comorbidity burden are significant predictors of mortality in this cohort. The demographics and clinical characteristics of the study population are displayed in Table 1.
Table 1
Demographics and clinical characteristics of the study population
The analysis of clinical outcomes based on gender revealed several statistically significant differences between male and female patients in various health parameters. Notably, the p-values indicate significant differences in the distribution of COVID-19 variants among genders, with females predominantly having the Alpha variant (p=0.005) and males displaying no significant variant distribution. Furthermore, the presence of fever was significantly more common in males without fever (p=0.017) and dyspnea was more prevalent in females without the condition (p=0.016). Additionally, vaccination status showed a significant difference, with a higher proportion of fully vaccinated males compared to females (p=0.007). However, no significant differences were observed in cough frequency, gastrointestinal symptoms, altered mental status, and the CCI, indicating similar morbidity profiles regardless of gender. The mean ages were also comparable, suggesting that age did not significantly influence the observed differences in clinical outcomes between genders (Table 2).
Table 2
Comparison of characteristics by gender
In the univariable Cox regression analysis, age (HR=1.039; 95% CI: 1.003–1.007, p=0.036), CCI (HR=1.231; 95% CI: 1.050–1.444, p=0.010), ferritin (HR=1.000; 95% CI: 1.000–1.001, p=0.044) and serum albumin (HR=0.943; 95% CI: 0.895–0.993, p=0.027) were identified as predictors of in-hospital mortality (Table 2), but in the multivariable Cox regression analysis none of these factors was independently associated with in-hospital mortality (Table 3).
Table 3
Univariable and multivariable Cox regression analysis (outcome: in-hospital mortality)
[i] ALP: alkaline phosphatase. ALT: alanine aminotransferase. AST: aspartate aminotransferase. CCI: Charlson comorbidity index. CRP: C-reactive protein. Fer: ferritin. FIB: fibrinogen. GGT: gamma glutamyl-transferase. Hb: hemoglobin. Hct: hematocrit. HR: hazard ratio. IGs: immature granulocytes. LDH: lactate dehydrogenase. Lym: lymphocytes. Neu: neutrophils. PLTs: platelets. WBC: white blood cell.
In the univariable Cox regression analysis, age (HR=1.034, 95% CI: 1.001–1.067, p=0.044), CCI (HR=1.261; 95% CI: 1.061–1.498, p=0.009), and serum albumin (HR=0.947; 95% CI: 0.902–0.994, p=0.029) were identified as predictors of 90-day mortality (Table 3), and in the multivariable Cox regression analysis, only serum albumin (HR=0.949; 95% CI: 0.903–0.998, p=0.042) was independently associated with 90-day mortality (Table 4).
Table 4
Univariate and multivariable Cox regression analysis (outcome: 90-day mortality)
[i] ALP: alkaline phosphatase. ALT: alanine aminotransferase. AST: aspartate aminotransferase. CCI: Charlson comorbidity index. CRP: C-reactive protein. Fer: ferritin. FIB: fibrinogen. GGT: gamma glutamyl-transferase. Hb: hemoglobin. Hct: hematocrit. HR: hazard ratio. IGs: immature granulocytes. LDH: lactate dehydrogenase. Lym: lymphocytes. Neu: neutrophils. PLTs: platelets. WBC: white blood cell.
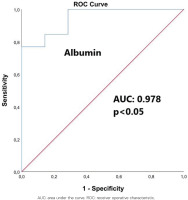
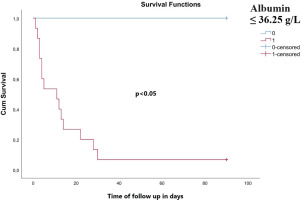
ROC analysis revealed that albumin levels served as a significant predictor of 90-day mortality, as depicted in Figure 1. A value of serum albumin ≤36.25 g/L predicted 90-day mortality with 100% sensitivity and 83.3% specificity (AUC=0.978, 95% CI: 0.955–0.999), negative predictive value 85%, positive predictive value 100% and accuracy 92.5%. Kaplan-Meier survival analysis based on cut-off values for albumin (≤36.25 g/L and >36.25 g/L) revealed a worse survival in subjects with an albumin value ≤36.25 g/L (log-rank test for trend, p<0.05) (Figure 2).
Figure 2
Kaplan-Meier survival curve. A significantly worse survival was observed for patients with an albumin value ≤36.25 g/L (log-rank test for trend, p<0.05)
Of note, when comparing the prevalence periods of different variants regarding the rates of in-hospital and 90-day mortality, there was only a statistically significant difference in in-hospital mortality between the delta variant predominance period and the alpha variant predominance period (HR=0.486; 95% CI: 0.238–0.994, p=0.048, data not shown).
DISCUSSION
According to a systematic review of COVID-19 characteristics in nursing home residents from the initial pandemic waves, the most common symptoms were fever and cough, followed by hypoxia and altered mental status11, while in our cohort of elderly nursing home residents, the most common symptoms were dyspnea and fever, followed by altered mental status and cough. In the 17 studies of the systematic review that evaluated mortality, the mortality rate of the patients was 40.2% during the observation periods11, while in our study, the total mortality during the observation period was higher (62%).
It has been reported that the risk of mortality in the period when the omicron variant was prevalent was reduced compared with the delta variant predominance period due to booster vaccinations, especially in the vulnerable nursing home population, and due to changes in therapy for COVID-1917. However, in that study, this difference did not reach statistical significance (HR=0.680; 95% CI: 0.440–1.040; p=0.076)17. In our study, there was only a statistically significant difference in in-hospital mortality between the delta variant predominance period and the alpha variant predominance period. Moreover, full vaccination did not seem to affect mortality rates. These discrepancies between our study and other studies published in the literature could be explained by different sample size, study settings, methodological approaches, and population characteristics.
One factor that may shed light on nursing home residents’ increased risk of COVID-19-related mortality is their pronounced inability to meet their lower and higher level requirements. For example, accumulating research has connected cognitive impairment to a decline in individuals’ ability to meet their physical and psychological requirements. Given that 47.8% of nursing home patients have dementia, whereas only around 33% of community-dwelling older people have the condition18,19, there are significant issues to consider. Furthermore, in comparison to community-welling older persons, nursing home patients have much more restricted physical access to family, friends, and acquaintances, the majority of whom are pillars of their social support system20,21. Family and friends function as informal caregivers in addition to being a primary source of social support21. This social interaction promotes companionship as well as human interconnection and relationship, which aids in satisfying the wants for love and belonging, esteem, and self-actualization22. While community-dwelling counterparts as well as nursing home residents have limited access to their key social contacts as a result of COVID-19 and subsequent social distancing mandates, the situation is significantly worse for nursing home residents23. Available research suggests that during COVID-19, virtually all nursing facilities barred outside visitors, essentially depriving these residents of a critical social support component24. Elder neglect and abuse have become increasingly concerning societal phenomena in the aftermath of COVID-19, where disturbing instances of spousal abuse of older persons have emerged in both home settings and nursing facility facilities25-27. While elderly neglect and abuse are health inequalities that older people confront throughout countries, this health disparity is particularly evident for nursing home residents, owing to their higher deficiency in self-care capacities and access to social assistance28. This discrepancy in health obviously increases nursing home residents’ vulnerability to the pandemic, which may explain the elevated COVID-19 mortality rates found in these groups.
Several factors, such as older age, functional and cognitive impairment, and specific comorbidities, have been reported as prognosticators of mortality in nursing home residents hospitalized due to COVID-1929-31. More specifically, dementia, renal function impairment, and Parkinson’s disease have been linked to increased mortality due to COVID-19 in these patients32. In addition, male gender has been recognized as a prognostic factor for mortality in nursing home residents with COVID-191,30. In our cohort study of elderly nursing home residents, we did not observe any differences in mortality between the two genders.
Several negative clinical consequences, including increased disability, morbidity, mortality, and declining quality of life, can result from malnutrition in elderly nursing home residents33,34. Decreased levels of albumin in individuals with COVID-19 could indicate heightened consumption stemming from tissue damage and increased metabolic activity, a common nutritional marker. A recent investigation found no correlation between frailty, nutritional condition, or grip strength and the occurrence or severity of COVID-19 among residents in nursing homes35.
To the best of our knowledge, our study is the first to demonstrate the association of serum albumin with unfavorable outcomes in elderly nursing home patients hospitalized due to COVID-19 pneumonia, indicating the important role of the nutritional and inflammatory states of these patients in the course of the disease. Existing literature refers to the role of albumin in patients with COVID-19 in general and it has been reported that low albumin levels are associated with poor outcomes in COVID-19 patients36.
Limitations
While the current study benefits from comprehensive clinical data and outcomes of elderly nursing home residents hospitalized with COVID-19 pneumonia over an extended period of the pandemic, it is important to acknowledge its limitations. Firstly, it was a single-center study with a relatively small participant pool and lacked a control group. Additionally, the study did not assess other inflammatory biomarkers or functional indicators that may be relevant to COVID-19 outcomes. The analysis did not account for the medication use for both COVID-19 and underlying conditions, nor did it provide detailed data on the causes of death for deceased individuals.
Furthermore, any association between nursing home residency and fatal COVID-19 outcomes may not apply to individuals treated as outpatients, as the study only included hospitalized patients. This study is also limited by potential residual confounding and limited generalizability to other countries or settings. One other limitation of this study is the potential issue of multiple statistical testing. With almost 30 parameters statistically tested, there is an increased risk of Type I errors, where some significant findings may occur by chance alone. Specifically, with this number of tests, it is expected that 1 to 2 significant findings could be due to random variation. To address this, we acknowledge this limitation in our study and suggest that the significant findings be interpreted with caution. Future studies should consider adjusting for multiple comparisons to minimize this risk.
Finally, it is important to note that the study did not individually identify viral variants for each patient. Instead, variants were assigned based on the predominant variant present at the time of SARS-CoV-2 diagnosis. These limitations warrant cautious interpretation of the study findings and highlight areas for further research and consideration in future studies.