INTRODUCTION
Venous thromboembolism (VTE) [i.e. pulmonary embolism (PE) and deep venous thrombosis (DVT)] is the third leading cause of cardiovascular mortality1. PE incidence and prevalence have increased in recent years, possibly due to improvements in diagnostic work-up, the increased awareness about PE and the elevated frequency of VTE risk factors in the general population2,3. PE management has changed in recent years due to the evolution of new drug therapies. Direct oral anticoagulants (DOACs) have largely replaced vitamin K antagonists (VKA) in the long-term treatment of PE patients, mainly due to the improvement of bleeding profile and the lack of need for regular dose monitoring4.
PE is a significant public health problem and is associated with a major financial burden for healthcare systems, patients and governments5,6. VTE is associated with substantial morbidity and mortality among hospitalized patients7 and leads to increased length of hospitalization, while, in the majority of patients, outpatient anticoagulant treatment is required. According to previously published reports8, the cost for VTE has three main aspects: cost of hospitalization, cost for outpatient management, and annual all-cause healthcare cost.
Few studies have estimated the financial burden of PE in Greece. To our knowledge, there are scarce data in the international literature that report on the overall burden of PE on public funds. To our knowledge, in the case of Greece, there are no published data concerning the financial burden of PE. In the present study, we aimed to estimate for the first time the PE outpatient pharmaceutical cost in Greece with the use of real-world data for the years 2013–2017.
METHODS
For the present study we used data provided by the National Organization for the Provision of Health Services of Greece (NPHO) concerning the electronic prescriptions that were issued during the period 2013–2017 with a diagnosis of PE. NPHO is a health insurance fund under Public Law and its operation began in January 2012 with the merger of the individual insurance funds of Greece. NPHO covers more than 90% of the Hellenic insured population. Nowadays, it is under the supervision of the Ministry of Health9. The health administrative data for Greece are collected by the Unit of Control and Processing of Prescriptions (KMES) of the Drug Management Section of NPHO which collects all the electronic prescriptions submitted to the system by all the pharmacies10. The administration of NPHO provided us with anonymized data (approved decision by the president/protocol number C31/906/22.06.2013) according to the national legislation on the Protection of Individuals with regard to the Processing of Personal Data. NPHO provided us with the number of prescriptions per patient for the years 2013–2017 with a diagnosis of PE (ICD-10 code I26 and ICD9 code 415). E-prescribing penetration in the Hellenic system is considered above 95% for outpatients10. A sub-analysis of the available data from NPHO has been recently reported2.
Since the datasheet from NPHO did not include the specific drug therapy of each patient, we obtained information from 200 random consecutive patients that were followed up by the Pulmonary Embolism Outpatient Clinic of the University Hospital of Larissa, in order to provide an estimation of the pharmaceutical cost for PE. Patients were enrolled by consecutive sampling. There were no restrictions on the time since PE diagnosis. Since the data received by the NHPO included patients at various time points after diagnosis, we included patients with newly diagnosed PE and patients with a history of PE during their follow-up. For the latter, we included data from all the time course of their disease (i.e. anticoagulants received shortly after discharge and during long-term follow-up). The collected data included the active substance the patients received throughout the course of their disease (as outpatients), the dosage and the duration of their treatment (i.e. from the diagnosis until the stop date or until the inclusion of the patient in the study); the data were used in order to generate treatment histories (outpatient treatment). The pharmaceutical cost reimbursed from NPHO for each patient was recorded as well. These collected data were used in tandem to the data provided by NPHO to estimate the average cost of each anticoagulant per patient as well as the pharmaceutical cost for the outpatient therapy of PE in Greece. Drug reference costs were drawn by the Hellenic National Drug Administration11.
RESULTS
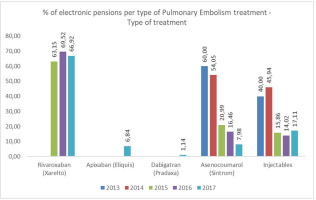
We included 200 random patients from the outpatient PE clinic with a mean age of 62.68 ± 15.70 years (55% females). All of the patients were hospitalized for PE and 5% required thrombolysis. The relative percentage of anticoagulant distribution is presented in Figure 1. In the years 2013 and 2014 (where DOACs were not available in Greece) 60% and 54.1% of the patients received acenocumarol and 40% and 45% received injectables, respectively.
In 2015 (when apixaban was not available in the Greek market for PE), 63.2% received rivaroxaban, 21% injectables and 15.9% acenocumarol. In 2016, 69.5% received rivaroxaban, 16.5% injectables and 14% acenocumarol. In2017, we observed that the anticoagulants received were mainly DOACs (rivaroxaban, apixaban, and dabigatran), followed by injectable anticoagulants (enoxaparin, nadroparin, tinzaparin, fondaparinux) and acenocumarol. In 2017, 66.9% received rivaroxaban, 6.8% apixaban, 1.1% dabigatran, 17.1% injectables and 8% acenocumarol specifically. The demographics (age and gender distribution) of the research sample are presented in Supplementary file Table S1.
Insurance coverage in Greece is as follows: patient co-payment is set at 0%, 10% and 25%, corresponding to 100%, 90% and 75% reimbursed from NPHO. The percentage of the co-payment per disease category is affected by the severity and duration of a disease according to a Ministerial Decree9.The relative percent of the pharmaceutical cost reimbursed from NPHO in our cohort of PE patients per year is presented in Table 1. For the whole study period, 94% of patients were reimbursed by 75% of the cost by NPHO, 1% of patients were reimbursed by 90% and 5% of patients were reimbursed by 100%.
Table 1
Relative percent of the pharmaceutical cost for PE reimbursed by NPHO for the years 2013 to 2017
| Percent reimbursed | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|
| 100 | 2.0 | 2.0 | 4.6 | 5.5 | 11.0 |
| 90 | 2.0 | 1.5 | 0.6 | 0.8 | 0.0 |
| 75 | 96.0 | 96.5 | 94.8 | 93.7 | 89.0 |
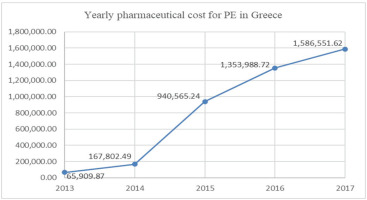
Taken together with the data from the 101426 electronic prescriptions for the years 2013–2017, we estimated the pharmaceutical cost of PE therapy in Greece. The cost for the treatment of outpatients with PE was estimated at €65910 in 2013 and increased to €1585552 in 2017 (Figure 2).
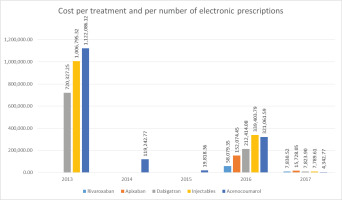
The estimated cost of PE per medication for the years 2013–2017 is presented in Figure 3. In 2013, the outpatient pharmaceutical cost of PE was €58079 for injectables and €7851 for acenocumarol. In 2014, the cost was €152075 for injectables and €15728 for acenocumarol. In 2015, the cost was €720327 for rivaroxaban, €212414 for injectables and €7824 for acenocumarol. In 2016, the cost was €1006795 for rivaroxaban, €339404 for injectables and €7790 for acenocumarol. In 2017, the cost of rivaroxaban was €1122086, the cost ofapixaban was €119243, the cost of injectables was €321062, the cost of dabigatran was €4343 and the cost of acenocumarol was €19818.
DISCUSSION
In the present analysis, we investigated the annual cost of the outpatient pharmaceutical treatment for PE in Greece for the years 2013–2017. The data obtained by the statutory health insurance organization of Greece taken together with the information derived by the PE outpatient Clinic of the University Hospital of Larissa provide important insights about the outpatient pharmaceutical cost as well as the associated reimbursed cost of PE. To our knowledge, this is the first study reporting data on PE drug cost in Greece. Our data are representative of the disease drug cost in PE since NPHO analyzes data that account for more than 90% of the country’s insured population while the e-prescribing penetration in Greece is above 95% for outpatients10. According to our results, the newer oral anticoagulants have a substantial financial burden for the treatment of PE while others have demonstrated that they require fewer laboratory visits, therefore they may reduce the additional costs of the funds as reported by other researchers12-15. Interestingly, rivaroxaban is associated with an additional acquisition drug cost estimated at €263 for PE while the standard of care (i.e. injectables followed by VKAs) is linked to a €64 additional cost of monitoring and €133 additional cost for adverse events5.
PE, along with DVT, significantly burden healthcare systems16. We observed that the estimated pharmaceutical cost of PE rises throughout the years studied, from €65910 in 2013 to €1586552 in 2017. Others have previously reported an increased annual health plan cost for services related to VTE throughout the years 1998–2004 to 2008–2011 in the USA17. The rise in the pharmaceutical cost of PE may be partly attributed to the increase in PE incidence in the Greek population in recent years3 that may be due to the enhanced incidence of VTE risk factors and the improvement in the diagnostic algorithms and methods. Additionally, patients that require extended treatment following acute PE have been well characterized while the improved safety of DOACs over VKA may have contributed to their increased use. Another possible explanation for the increase in the pharmaceutical cost of PE may be the increase of reimbursement across the years studied, as depicted in Table 1.
PE treatment has evolved in the recent years and DOACs have substantially prevailed over VKAs and injectables in the outpatient therapy of PE patients. DOACs were developed in the past 20 years6; rivaroxaban, apixaban and edoxaban block the activated factor X (Xa) and dabigatran exetilate inhibits thrombin18. PE acute treatment focuses on anticoagulants with rapid onset of action like injectable agents (LMWH or fondaparinux) or DOACs. Usually, DOACs are administered for at least 3 months19. VKAs are nowadays used when contraindications to DOACs are present (e.g. severe renal or liver failure, concomitant use of interfering drugs, antiphospholipid syndrome) and require frequent monitoring with blood sampling and physician counselling in order to achieve therapeutic effect4. The choice of anticoagulant therapy may be partly influenced by the patient’s history (including comorbidities that may serve as risk factors). Injectables are not often used in the outpatient treatment of PE and their applications include cancer patients, patients with relapses while on oral agents, etc.20. In addition to drug therapy, several mechanical approaches have been used in order to provide immediate reperfusion (e.g. percutaneous thrombectomy) or to limit the use of anticoagulants in cases of adverse events or relapses (i.e. inferior vena cava filters)21. For both approaches, the cost-effectiveness is not determined. However, recent data suggest that the cost of interventional therapies, such as catheter-directed treatment, may increase in the following years22. We observed that the majority of outpatients received DOACs, with most of them receiving rivaroxaban and apixaban. Similar prescription patterns were observed in a French registry where rivaroxaban and apixaban were used at the time of their discharge in 93.6% and 6.4%, respectively, of patients discharged with DOACs23. DOACs appear to have advantages related to their rapid initiation of treatment, frequent follow-up, low number of side effects and prespecified drug dosage24-26. However, when assessing the indirect cost of DOACs, one has to take into account the potential cost that may occur in the case of adverse events that require the use of their reversal agents (idarucizumab, andexanet alfa and ciraparantag)27.
Our results indicate that DOACs account for the majority of the outpatient pharmaceutical cost for PE in Greece, rivaroxaban treatment having the higher amount, followed by apixaban and dabigatran exetilate. Treatment with VKAs, although having a lower average monthly cost per patient, has been associated with higher health costs than DOACs due to the fact that patients must regularly follow a series of tests and physician visits, therefore the total cost of treatment rises significantly23. When estimating the outpatient cost of PE, one must take into account indirect costs related to productivity lost which may be considerable, especially in the first months following PE28. Studies have suggested that each incident case may account for the loss of approximately 1.2 years of heathy life8.
In order to obtain the estimated pharmaceutical cost burden of PE for Greece, the charge of the insurance funds, and not the financial burden of the patients, were calculated per electronic prescription. It turned out that the total charge of NPHO for the 101426 electronic prescriptions for the years 2013–2017 amounts to €4114818 and we estimated that rivaroxaban is the first in sales with a total cost of €2849209, followed by apixaban with a total cost of €119243, injectables with a total cost of €1083033, while dabigatran has a total cost of €19816. Studies that previously examined PE related cost, have shown that direct Xa inhibitors provide a consistent dose regimen with fewer laboratory tests requirements and may represent an option with a lower overall cost29-35. A 2013, study also showed that hospitalized patients have significantly lower hospital costs by $2245 per patient due to the shorter hospital-stay days associated with DOACs. In the United Kingdom, studies have shown that rivaroxaban may be the most cost-effective treatment versus the LMWH/VKA approach with £8677 per quality-adjusted life-year (QALY) for patients with DVT and £7072 per QALY for patients with PE36. In 2016, a comparison between the cost of DOACs and that of warfarin was made and it was observed that the overall cost of warfarin was higher37.
No comparison can be done between the data represented by the present research and the data of corresponding research of data mining due to the lack of similar studies in Greece. Existing research suggests that DOACs are a cost-effective treatment for PE. For Greece, the only research that has been performed on e-prescriptions concerns chronic obstructive pulmonary disease (COPD)10 and emphasizes the potential role of secondary analysis of existing administrative data for health policy.
Limitations
This study has several limitations. The retrospective nature of the study design may result in limitations and caveats that are associated with secondary data sources. Additionally, we did not estimate indirect costs associated with PE that may be associated with a substantial economic burden. We did not have direct available data from the NPHO for the specific anticoagulant prescribed. Moreover, we combined data from NPHO with data from a single center outpatient PE clinic, thus, one may speculate that this may limit the extrapolation of the results to the Greek population. However, to our knowledge, this is the only specialized outpatient clinic in PE in Greece that regularly follows up patients from a large geographical region with approximately 730000 population38. In addition, we cannot estimate whether the cost was related to acute events, or relapses, and we do not report data concerning the etiology of VTE events (cancer, etc.) or clinical outcomes associated with therapy (i.e. bleeding events). In our study, DOACs were associated with an increased direct financial burden; however, previously published data suggest that they require fewer laboratory visits that may reduce the additional costs of the funds12-15. Unfortunately, our study was not designed to address this.
CONCLUSIONS
The analysis based on the existing administrative data provided by the NPHO, estimates the outpatient pharmaceutical cost for the treatment of PE for Greece for the years 2013–2017. That cost seems to be increasing throughout the years and is mainly attributable to DOACs, possibly due to the large penetration of those drugs for PE management.