INTRODUCTION
Tuberculosis (TB) is one of the biggest health problems in the world and a major cause of death worldwide. Tuberculosis is an infectious disease caused by Mycobacterium tuberculosis and present in all countries and age groups. In 2022, WHO reported that there were 10.6 million cases of TB and 1.1 million people died globally. According to the WHO, there were approximately 1.1 million cases of TB in Indonesia1. The initial empirical treatment of tuberculosis, starts on a 4-drug regimen (rifampicin, pyrazinamide, isoniazid, and ethambutol or streptomycin). The high incidence rate of TB is caused by ineffective treatment which is correlated with poor adherence to the TB treatment2. Currently, the duration of therapy for the initial phase of drug-susceptible tuberculosis (DS-TB) is 6 months, which is much longer compared to other infectious respiratory diseases1. Poor adherence to TB treatment was associated with an increased risk of adverse outcomes. Moreover, another concern for TB therapy is the increase of rifampicin-resistant TB with approximately 410000 cases of multi-drug resistant/rifampicin-resistant TB globally3. Therefore, tuberculosis treatment needs to be carried out in a shorter and easier manner than the current six-month daily regimen, thereby increasing adherence and potentially reducing adverse drug effects1,4,5.
Rifapentine, a cyclopentyl ring-substituted rifamycin with a longer half-life than rifampicin, is active against M. tuberculosis. Therefore, the potential of rifapentine for enhanced treatment has been explored. The US Food and Drug Administration approved rifapentine for the treatment of active tuberculosis, at a dose of 1200 mg weekly in combination with other antituberculosis drugs6,7. Preclinical research on mouse models has demonstrated that three months of daily rifapentine therapy is sufficient to cure patients. In tuberculosis treatment, the rifapentine-based regimen for 4 months containing moxifloxacin was not inferior to the standard regimen for 6 months8,9. The combination of rifapentine and moxifloxacin was active in a murine tuberculosis model. It can achieve stable recovery after 3 months of treatment10.
According to the newest update of WHO guidelines for TB, rifapentine is used for a 4-month regimen with the combination of isoniazid, rifapentine, moxifloxacin, and pyrazinamide (2HPMZ/2HPM)11. However, these recommendations have moderate evidence and there are no systematic reviews or meta-analyses regarding the use of rifapentine in the initial and/or continuation phases of DS-TB. This study aimed to determine the efficacy and safety of a rifapentine-containing regimen for DS-TB. This study is expected to be a reference for shorter TB therapy in order to succeed in universal TB treatment.
METHODS
Data sources and selection
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) was used to organize the review11. The relevant studies were searched from the databases of PubMed, Cochrane, Science Direct, Google Scholar, Epistemonikos, Proquest, and clinicaltrial.gov. Article selection based on PICOS framework such as: drug sensitive tuberculosis (Patients), rifapentine containing regimen (Intervention), no comparison OR previous regimen (RHZE) (Compare), sputum conversion rate OR adverse effect (Outcome), and [RCT] OR [CT] OR [observational studies] (Study). We exclude all studies for which : 1) the full text was not accessible, 2) the research was still ongoing, 3) the subjects were classified as a latent or drug resistant TB individuals, and 4) the regimen was for extensive phase or preventive therapy. This review is registered as CRD42023464003 PROSPERO, International prospective register of systematic reviews (URL: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023464003).
Data extraction and quality assessment
We extracted basic data such as: 1) the corresponding author of the selected study, 2) year of publication, 3) country in which the study was conducted, 4) design of study, 4) sample size, 5) dose of rifapentine regimen, 6) regimen, 7) comparison, 8) duration of treatment, 9) number of converted or not converted sample, and 10) reported adverse effects. Data extraction and quality assessment was performed independently by five investigators (RSD, AM, FS, PM, AH) using the Cochrane risk-of-bias tool (RoB 2) for randomized trials. Disagreements were resolved by consensus, or involving supervisors (NR) when consensus was not reached. The primary outcome of this study used risk ratios (RRs) with 95% confidence intervals (CIs). When appropriate, we pooled data from the included trials in meta-analyses.
Statistical analysis
The study efficacy outcome in this review was defined by the sputum rate conversion to negative on the rifapentine regimen for DS-TB at the end of the intensive phase (8 weeks) and compared to the previous regimens (RHZE regimen), the study’s primary and secondary outcomes, respectively, while safety analysis of the rifapentine regimen was the study’s secondary endpoint. The statistical analysis engine is then used to analyzed all of the extracted data. The Review Manager (RevMan) 5.4 application is used to implement statistical analysis for this meta-analysis. A statistical machine determined the value of OR and RR. Chi-squared values and the inconsistency index (I2) were used to assess the degree of heterogeneity among the included studies. There is heterogeneity between the studies if the chi-squared value has a p<0.05. When an overall effect result has a total p≤0.05 and a 95% confidence interval (95% CI) of OR or scope on the overall does not cut line 1, it is considered statistically significant. Because we utilized a random effect model, the conclusion might be applied to other research, outside those that were included.
RESULTS
Study selection
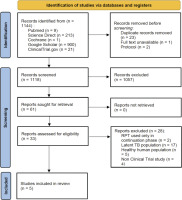
Through the use of several databases, we obtained 1144 articles. Six studies were deemed appropriate for further evaluation after duplicate publications were removed by abstract screening, leaving 5 studies that satisfied the eligibility requirements (Figure 1)7,12-15.
The characteristics of the studies
The characteristics of included studies are depicted in Table 1. All included studies used an RCT study design in the intensive phase or intensive + continuation phase of DS-TB patients. Overall, this review included a total of 3655 study subjects which consists of 2377 subjects from the rifapentine group and 1278 subjects from the control group. The sample size varied widely, depending on the method of data collection and database used, and ranged from 50% to 74.5% from total of 121 to 2516 patients. All included studies compare the use of rifapentine or rifapentine + moxifloxacin with a standard DS-TB drug/RHZE regimen. The average duration of administration and duration for the intervention is once per day for 8 weeks in the intensive phase and 9 weeks in the continuation phase.
Table 1
Characteristics of included studies
| Author Year | Country | Design | RPT/Total sample n/N (%) | Patients age (years) | Males n (%) | HIVpositive n (%) | RPT dosage | RPT arm regimen | Control arm regimen | Administration and duration |
|---|---|---|---|---|---|---|---|---|---|---|
| Dorman et al.7 2021 | Brazil, China, Haiti, India, Kenya, Malawi, Peru, South Africa, Thailand, Uganda, United States, Vietnam, and Zimbabwe | Multi-center | 1685/2516 (66.9) | ≥12 | 1670 (71) | 194 (8) | RPT 1200 mg and RPT 1200 mg + MOX 400 mg | RPT + H + Z + E and RPT+ H + Z + MOX | R + H + Z + E | Once per day for 8 weeks (intensive phase) and 9 weeks (continuation phase) in RPT group vs 8 weeks (intensive phase) and 18 weeks (continuation phase) in control group |
| Conde et al.15 2016 | Brazil | Multi-center | 62/121 (51.2) | ≥18 | 83 (69) | 0 | RPT 7.5 mg/Kg + MOX 400 mg | RPT + H + Z + MOX | R 10 mg/Kg + H + Z + E | Once per day for 8 weeks (intensive phase) in each group |
| Dawson et al.14 2015 | South Africa | Single-center | 105/153 (68.6) | ≥8 | 116 (75.8) | 23 (15) | RPT 450 and 600 mg | RPT + H + Z + E | R 600 mg + H + Z + E | Once per day for 8 weeks (intensive phase) in each group |
| Dorman et al.13 2015 | North America, Africa, South America, Asia, and Europe | Multi-center | 249/334 (74.5) | ≥19 | 230 (68.9) | 26 (7.8) | RPT 10, 15, and 20 mg/Kg | RPT + H + Z + E | R 10 mg/Kg + H + Z + E | Once per day for 8 weeks (intensive phase) in each group |
| Dorman et al.12 2012 | North America, South Africa, Uganda, Spain, Brazil, Peru, and Vietnam | Multi-center | 276/531 (51.9) | ≥18 | 260 (66.8) | 42 (11) | RPT 10 mg/Kg | RPT + H + Z + E | R 10 mg/Kg + H + Z + E | Once per day for 8 weeks (intensive phase) in each group |
Quality of evidence and risk-of-bias
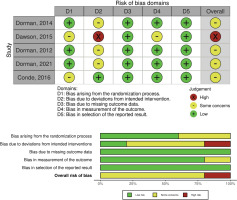
This review uses Cohrane’s risk-of-bias 2 (RoB 2) tool in RevMan 5.4.1. Out of the five studies, four were found to have a medium-high risk-of-bias in terms of deviation from intended intervention, either because the participants or personnel are aware that they are in a trial. These studies employed open-label designs potentially impacting the assessment of results. Furthermore, studies exhibited a medium risk, two of them related to randomization process and one related to measurement outcome, poorly explained in the method. However, all studies demonstrated a low risk-of-bias in terms of missing outcome data and selection of the reported result (Figure 2).
Efficacy
Sputum conversion for rifapentine
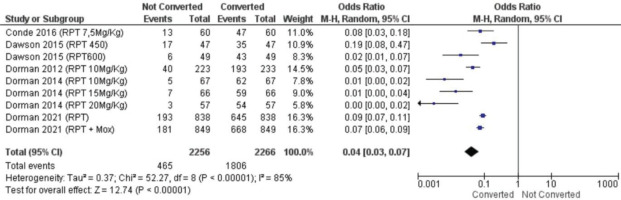
We performed two statistical tests of meta-analysis, specifically to evaluate efficacy of rifapentine for DS-TB and sputum conversion rate rifapentine compared to a standard regimen for DS-TB. The analysis suggests rifapentine superior in converting the sputum significantly (OR=0.04; 95% CI: 0.03–0.07) (Figure 3). All data included in Figure 3 appeared heterogeneous between studies (p<0.00001). This could be due to differences in treatment doses between studies as well as regimens, resulting in heterogeneous between-study data.
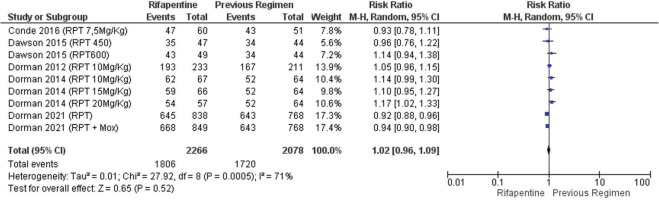
Sputum conversion was relatively higher in patients receiving rifapentine-containing regimens than in those with standard regimens, though the result was not statistically significant (RR=1.02; 95% CI: 0.96–1.09) (Figure 4). The data in Figure 4 were heterogeneous between studies (p<0.0005), which could similarly be due to dose and regimen differences in the rifapentine arm, as well as dose and regimen differences in the comparison arm.
Sputum conversion for rifapentine regimen with 1200 mg dosage
The dosage of rifapentine in each included study was diverse. Thereby, we had an analysis of the sputum conversion rate in rifapentine in which the dosage was stated to be 1200 mg7,13. The analysis suggests that DS-TB patients with rifapentine regimen had a 56-times greater conversion rate (p<0.0001). The data in Supplementary file Figure 1 are heterogeneous between studies.
Subgroup analysis was performed to compare the rifapentine regimen with 1200 mg to a previous regimen (RHZE) for DS-TB. The first subgroup, the regimen without moxifloxacin, suggests a non-significant yet non-inferior result to the previous regimen (Supplementary file Figure 2) (p=0.48), and the data are heterogeneous between studies. The second subgroup, comparison of the rifapentine with moxifloxacin regimen to the previous regimen group, suggests that it had a greater conversion rate than the previous regimen arm with a significant result (p=0.010). Overall, the analysis of sputum conversion for rifapentine with 1200 mg dose had an insignificant rate of sputum conversion rate difference to previous regimens.
Safety analysis
The included studies reported adverse events (AEs) grades 1–5 according to The National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), including blood and lymphatic system disorders, cardiac disorders, gastrointestinal disorders, hepatobiliary disorders, etc16. The results of the meta-analysis showed that the rifapentine-containing regimen did not increase the incidence of AEs compared to the standard regimen, with no statistically significant difference in the total AEs of the two groups (RR=0.96; 95% CI: 0.78–1.18, p=0.70) (Supplementary file Figure 3). The results of heterogeneity test (p=0.008, and I2=62%) suggested that there was some heterogeneity among the included studies. The data in Supplementary file Figure 3 are heterogeneous between studies. This may be due to differences in regimens, resulting in different side effects.
DISCUSSION
To our knowledge, this is the first systematic review and meta-analysis about the efficacy and safety of a rifapentine-containing regimen for DS-TB. This review sums up all the evidences across nations with a total 2377 subjects in the rifapentine arm group. Previous systematic reviews had shown the efficacy and safety of rifapentine and isoniazid for either preventive treatment or latent TB population17-19. The network systematic review of Imazu et al.20 on the short treatment duration of a rifapentine-containing regimen suggested that the rifapentine-containing regimen was statistically safer than other regimens for serious side effects such as hepatotoxicity and arthralgia while maintaining its efficacy, being not inferior to a standard regimen. However, this study did not specifically analyze the reduction of the TB treatment period to 4 months and still used a 6-month treatment period20. Conversely, our results demonstrate that the efficacy of rifapentine-containing regimen was not significantly different to that of the standard regimen (OR=1.02; 95% CI: 0.96–1.02). This indicates that the rifapentine-containing regimen can be given for the treatment of TB for a shorter duration but with the same efficacy as the standard regimen.
In this meta-analysis, all included studies did not report the incidence of post-intervention relapse and resistance at follow-up. The study by Alfarisi et al.21 reported that rifapentine has the same effectiveness as rifampin. Rifapentine was less hepatotoxic than rifampin, but rifapentine caused flu-like symptoms. Rifapentine has a higher potential for recurrence if the optimal dose is not used21. The study by Jindani et al.22 reported that the high-dose rifapentine regimen in a continuation phase has the potential to treat TB with a low relapse rate or resistance, with all of the rifapentine regimen combined with moxifloxacin. A review analyzed the shortened treatment regimen, either with moxifloxacin or gatifloxacin, and found that it did not increase the resistance, although they had relapse tendencies compared to a standard regimen, specifically for a population with HIV comorbidity and which lived in high intensity of transmission23.
Our risk-of-bias analysis of the included studies showed medium risk with some concern in the deviation in intended results. All of the studies employed an open-label design which potentially has the possibility for missing data, due to knowledge of assigned treatment and expectations of those involved in the trial24. The varying dose of rifapentine in each study and subgroup, may disrupt the efficacy and drug safety of rifapentine. Therefore, we had an additional subgroup analysis with a 1200 mg dose of rifapentine regimen group, and the regimen combined with moxifloxacin.
This review revealed that the dose of 1200 mg in a rifapentine regimen group was not inferior in terms of sputum conversion rate compared to the RHZE regimen. Several studies are relevant to our review which underlines the optimal dose of rifapentine. Based on the study protocol in the Chinese population, the optimal dose regimen containing rifapentine and moxifloxacin for DS-TB is a rifapentine dose of 10 mg/kg, 15 mg/kg, and 20 mg/kg, with a maximum dose of 1200 mg, and a dose of moxifloxacin of 400 mg25. A study protocol for phase 3 clinical trial was also proposed by Dorman et al.7 regarding high-dose rifapentine with or without moxifloxacin for shortening treatment of DS-TB, with the optimal dose being 1200 mg, given every day for 4 months. The optimal dose of rifapentine (900–1200 mg) maximized the treatment efficacy based on pharmacokinetic/pharmacodynamic data26,27. Overall our review underlined the favorable outcome of the proposed regimen, but it was not statistically significant in both sputum conversion and AEs, compared to the standard regimen (6-month RHZE). This finding had a narrow range of 95% CI values, providing high certainty and more accuracy in the data.
The outcome of the study may alleviate the level of recommendation of rifapentine-containing regimen by WHO, which was previously classified as a conditional recommendation with moderate certainty of evidence. The shorter duration (4 months) of the rifapentine-containing drug regimen suggested by WHO may give a superior benefit of adherence and lower resistance, compared to past therapeutic suggestions10. Longer treatment times and the lack compliance of the current standard therapy (6 months) contribute to the increased trend of rifampicin-resistant tuberculosis, globally27,28. This may lead to treatment failure, increased mortality, and cause the development of drug-resistant disease.
Limitations
This review has some limitations. Regimen combinations in the included studies, in both the intervention and control group, and rifapentine dosage among participants, greatly varied and might have influenced when progression from infection to disease was noted. Therefore, the adverse effects appeared inconsistent across the studies. This review was limited to articles in English and in Indonesian; this might have resulted in the search for evidence missing high-quality articles published in other languages. Finally, the absence of cost and cost-effectiveness data in the included studies precluded the authors’ ability to conduct an economic evaluation of use of the rifapentine-containing regimen. WHO stated that the cost burden of rifapentine has curtailed the drug’s accessibility, although in a latent TB population, rifapentine regimen has more benefit in saving costs and improving health29. We further advise conducting a study on low heterogeneity regarding the efficacy and adverse effects, for the same dosage and duration recommended by WHO in 2020. The limitations in this study related to the inconsistency of adverse effects, publication language, and the absence of cost and cost-effectiveness of the proposed regimen, may be overcome by future research, which might have a level of evidence that could support the recent recommendation.
CONCLUSIONS
The findings of this systematic review and meta-analysis indicate that novel once per day rifapentine-containing regimen is effective and safe, with significantly shorter treatment duration compared to previous regimens (RHZE), for the treatment of DS-TB. These results further support the recommendations of the Guideline Development Group (GDG) meetings in 2021 and the World Health Organization (WHO) 2022, which advocate a 4-month regimen of isoniazid, rifapentine, moxifloxacin, and pyrazinamide, as the shorter treatment option for DS-TB. However, further studies are needed to determine the optimal dosing strategies and potential drug interactions associated with rifapentine-containing regimen.