INTRODUCTION
COVID-19 is an infectious disease declared as pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), as was designated by the World Health Organization in February 20201. The first cluster of cases was reported in the city of Wuhan, in China on the 31 December 2019 as ‘pneumonia of unknown cause’, later identifying the novel virus responsible for the disease. COVID-19 is a multisystem inflammatory syndrome with a rather wide variety of symptoms, the most common of which are fever, cough, fatigue, myalgia, headache, nasal obstruction/rhinorrhea, sore throat and loss of smell2, along with up to 32% of asymptomatic cases3. According to WHO, the disease severity classification includes 3 categories of patients, those with non-severe, severe, and critical disease. The last regards patients with acute respiratory distress syndrome (ARDS) and other life-threatening situations4.
ARDS is an acute, diffuse, inflammatory lung injury with acute hypoxemia, decreasing lung compliance and bilateral opacities. The damage to the alveolar-capillary membrane leads to increased permeability and subsequent interstitial and alveolar oedema, resulting in severe hypoxemia due to intrapulmonary shunting and V/Q mismatch5. Primary goal in treating ARDS is to improve patient ventilation. The improvement of ventilation during prone position is multifactorial; while in supine position, ventral transpulmonary pressure is greater than dorsal, resulting in overinflation of ventral alveoli and atelectasis of dorsal ones. On the other hand, prone position reduces ventral and dorsal transpulmonary pressure, making ventilation more homogeneous6. The application of positive end-expiratory pressure (PEEP) leads to more uniform pressure distribution, lung expansion and alveolar recruitment. In patients with ARDS in supine position, the heart and diaphragm compress the posterior lung parenchyma. Lung compression by both the heart and the diaphragm can be favorably affected by prone positioning, allowing previously non-ventilated lung regions to participate in the gas exchange7. At the same time, pulmonary perfusion remains distributed mainly to the dorsal lung regions. In other words, the gravitational distribution of pulmonary blood flow may be only minimally altered by prone position and the observed changes in gas exchange are primary due to changes in regional ventilation, thus improving overall alveolar ventilation/perfusion relationships8. Moreover, the reduction of hypoxic vasoconstriction in prone position decreases right heart afterload, resulting in a decrease in pulmonary resistance. Additionally, secretion drainage seems to be improved due to gravitational effect. Prone positioning combined with mechanical ventilation has shown significant improvement in oxygenation and ventilation9.
In order to avoid the progression of COVID-19 pneumonia to ARDS and in an attempt to improve outcomes at times of limited resources, even in the most advanced healthcare systems, many centers have applied prone position as a therapeutic supportive measure in non-intubated patients with COVID-19 and respiratory failure.
The aim of the current review is to summarize the evidence of the effect of prone positioning in patients with COVID-19 pneumonia not on invasive mechanical ventilation, based on published literature.
METHODS
In this review, a search was conducted in PubMed for eligible studies. The key search terms were: (prone position) AND (COVID-19). Only observational studies and controlled trials in English were included. Two authors screened article titles and abstracts for inclusion/exclusion criteria. Detailed inclusion and exclusion criteria are displayed in Table 1. Full text of the remained articles was assessed and all studies without control group comparisons were excluded. This research was conducted on April 2023.
Table 1
Inclusion and exclusion criteria
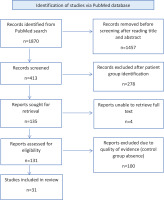
Regarding the exclusion criteria of each study, patients were not analyzed if they needed immediate intubation at admission or were already under mechanical ventilation, were hemodynamically unstable, were pregnant, had recent (in the last 30 days) abdominal surgery, were overweight with a body mass index over 30 kg/m², were unable to prone because of discomfort, were contraindicated to prone or had a do-not-intubate or do-not-resuscitate order. Additional exclusion criteria, were patients who were voluntarily discharged or referred to another hospital, were previously intubated due to COVID-19 AHRF, were unable to provide a consent form or to understand oral or written study information, subjects with incomplete clinical records, and specific conditions such as unstable fractures, intracranial hypertension, hypercapnia and terminal illness (less than 1 year life expectancy). The screening and selection process is displayed in Figure 1.
RESULTS
After inclusion/exclusion criteria were evaluated, a total of 31 studies were included in this review10-40. The main characteristics of the included studies are listed in Table 2.
Table 2
Study characteristics
| Authors Year | Location | Design | Enrollment period | Study population | Oxygen therapy | Prone protocol | COVID-19 category according to WHO of patients at enrollment | Outcomes | Results |
|---|---|---|---|---|---|---|---|---|---|
| Ehrmann et al.15 2021 | Canada France Ireland Mexico USA Spain | Prospective collaborative meta-trial of 6 randomized controlled open-label superiority trials | 2 April 2020 to 26 January 2021 | 1121 patients with COVID-19 AHRF (564 PP vs 557 SC) | HFNO | PP for as long as tolerated | Severe and Critical COVID-19 except for hemodynamically unstable P/F <300 mmHg | Treatment failure within 28 days of enrolment, defined as intubation or death | Lower incidence of intubation at day 28 in the intervention group (RR=0.86; 95% CI: 0.75–0.98), but same 28-day mortality rate (RR=0.87; 95% CI : 0.71–1.07) |
| Padrão et al.12 2020 | Sao Paolo, Brazil | Retrospective cohort study | 1 March to 30 April 2020 | 166 patients admitted to the ED as suspected COVID-19 case (57 PP vs 109 SC) | Supplemental oxygen with a flow rate ≥3 L/min | Prone position for at least 4 h in the first session | Severe and Critical COVID-19 except for hemodynamically unstable | Intubation rate up to 15 days after PP initiation | Not statistically significant difference between PP and control group (HR= 1.21; 95% CI: 0.78–1.88, p =0.39) |
| Jagan et al.10 2020 | Nebraska, USA | Retrospective study | 24 March to 5 May 2020 | 105 COVID-19 patients (40 PP vs 65 SC) | Not defined | Self-proning ≥1 h on ≥5 occasions/day and ≥1 h overnight | Category not | Need of ETI during standardized hospital stay | Risk of ETI was lower in PP after adjustment for SOFA score (AHR=0.30; 95% CI: 0.09–0.96; p=0.043) or APACHE II scores (AHR=0.30; 95% CI: 0.10–0.91, p=0.034). No prone patient died compared with 24.6% of patients who were not prone (p<0.001; number needed to treat =5; 95% CI: 3–8) |
| Ni et al.11 2020 | Wuhan, China | Prospective observational cohort study | 31 January to 15 February 2020 | 52 patients with severe COVID-19 (17 PP vs 35 SC) | Not defined | Prone position for at least 4 h/day for 10 days | Severe COVID-19 P/F ≤300 mmHg RR ≥30 breaths/min | Efficacy of early PP intervention on oxygenation improvement | PP resulted in improvement in SpO2/FiO2 (409; 95% CI: 86–733) and ROX index (26; 95% CI: 9–43) and decreased Borg scale (-9; 95 % CI: -15 to -3) |
| Zang et al.13 2020 | Beijing, China | Prospective single-center cohort study | 1 February to 30 April 2020 | 60 COVID-19 patients with severe hypoxia (23 PP vs 37 SC) | Not defined | PP for 10 min and 30 min | Severe COVID-19 | Improvement of hypoxia, CT imaging and survival | During PP, SpO2 increased from 91.09 ± 1.54% to 95.30 ± 1.72% (p<0.01) after 10 min, 95.48 ± 1.73% after 30 min (p<0.01), but no significant difference after 30 min compared with 10 min (p=0.58) |
| Jouffroy et al.19 2021 | Paris, France | Retrospective observational study | 20 February to 24 April 2020 | 379 COVID-19 patients admitted in ICU (40 PP vs 339 SC) | LFNO, HFNO, NIV | Prone position for 3–6 h twice/day | Critical COVID-19 | PaO2/FiO2 ratio, ABGs, survival and intubation rate at 28 days | Increase PaO2/FiO2 (p=0.004) and PaCO2 (p=0.005) in the intervention group while NS difference in survival (p=0.419) and intubation (p=0.178) rate |
| Barker et al.29 2022 | London, UK | Retrospective study | 26 March to 26 June 2020 | 20 COVID-19 Not defined patients (10 PP vs 10 SC) | Not defined | Prone position for as long as possible | Severe COVID-19 PaCO2 <45 mmHg | SpO2/FiO2 ratio recorded after each PP session | Only after the first PP episode, increase in SpO2/FiO2 ratio was observed (before, PP=152, IQR:135–185; after, PP=192, IQR: 156–234, p=0.04) |
| Ates et al.14 2021 | Ankara, Türkiye | Retrospective study | 15 August to 1 December 2020 | 124 patients (97 PP compliant vs 47 PP incompliant) | Oxygen therapy not needed | Six different positions were used in total (prone, left/right lateral decubitus, left/right swimmer’s, supine). SpO2 was assessed after 5 min in each position. Patients maintained the 2 positions with the better SpO2, switching in 6-h to 8-h intervals, with breaks according tolerance | Mild COVID-19 | Positioning duration, rate of ICU admission, anti-inflammatory treatment and length of hospital stay were assessed in compliant and incompliant with PP patients | Positioning duration was median 12 (3–20) vs 5 (2–16) in compliant and incompliant patients, while rates of ICU admission (7.2% vs 25.5%, p<0.001), anti-inflammatory treatment initiation (68% vs 97.9%, p<0.001) and length of hospital stay [5 (2–16] days vs 12 (3–20)] days, p<0.001) were significantly reduced in compliant with PP patients |
| Liu et al.22 2021 | Wuhan, China | Retrospective observational study | 22 January to 13 March 2020 | 29 patients (13 early PP vs 16 later PP), later defined as PP therapy after 3 days | LFNO | PP for ≥2 h in the morning, ≥2h in the afternoon and ≥6 h at night, total PP 10–14 h/day | Non-severe (mild) COVID-19 | PP duration and length of hospitalization | Early PP group showed significantly shorter total PP time (HR= -5.8; 95% CI: -9.45 to -2.14, p=0.006) and total length of hospitalization (HR= -11.03 95% CI: -14.62 to -7.45), p=0.000) |
| Kaur et al.20 2021 | USA | Collaborative meta-trial of six randomized controlled trials | 2 April 2020 to 26 January 2021 | 125 COVID-19 patients [92 early PP (PP initiated within 24 h of starting HNFC therapy) vs 33 late PP] | HFNO | Early PP (within 24 h of starting HFNC therapy) PP ≥1 h | Severe and Critical COVID-19 S/F <240 | 28-day mortality and intubation rate among patients that received early vs late APP | Lower mortality rate in early APP group (45% vs 26%, p=0.039), while NS difference in intubation rate p=0.58 |
| Jayakumar et al.17 2021 | Chennai, India | Multicenter feasibility randomized controlled trial | Not defined | 60 patients (30 PP vs 30 SC) | Not defined | PP ≥6 h/day | Severe and Critical COVID-19 P/F: 100–300 mmHg, PaCO2 <45 mmHg ≥4 L/min supplemental oxygen to maintain SpO2 ≥92% | Adherence to the protocol (PP for at least 6 h) in each group | 43 % protocol compliance |
| Hashemian et al.16 2021 | Tehran, Iran | Prospective study | 26 February to 25 April 2020 | 75 COVID-19 patients under NIV admitted in ICU (45 PP vs 30 SC) | NIV | PP of 30 min every 4 h, additional 30 min PP session if SpO2 <82% | Severe and Critical COVID-19 | Effect of PP in SpO2, PaO2/FiO2 and need for ETI | NIV combined with PP resulted in a significantly shorter length of ICU admission (8.6 vs 14.4, p=0.046) |
| Kharat et al.21 2021 | Geneva, Switzerland | Cluster randomized control trial | April to May 2020 | 27 COVID-19 patients (10 PP vs 17 SC) with LFOT | LFNO | PP for 12 h/day | Non-severe and Severe COVID-19 S/F >225 with LFNO | Effect of PP in oxygen needs | No statistically significant difference between two groups |
| Prud’homme et al.24 2021 | Marseille, France | Exposed/ non-exposed bicentric retrospective matched cohort study | 20 March to 20 April 2020 | 96 COVID-19 patients (48 PP vs 48 SC) | LFNO, HFNO | PP for at least 3 h/day for 3 days | Category not standardized | Upgrade in oxygen delivery method at day 14, defined as doubling of the initial oxygen supply | 31.2% of PP patients had an upgrading of oxygenation method vs 52.1% of the control group (p=0.038) |
| Vianello et al.27 2021 | Padua, Italy | Prospective cohort study | 1 November 2020 to 28 February 2021 | 93 COVID-19 patients under HFNC (50 PP vs 43 SC) | HFNO, NIV | PP ≥2 h twice/day | Severe COVID-19 | Effect of PP in ETI | PP was associated with clinical benefit and survival without escalation of therapy in 80% of subjects of PP group |
| Johnson et al.18 2021 | Utah, USA | Nonblinded pragmatic randomized controlled trial | 29 April to 5 August 2020 | 30 COVID-19 patients (15 PP vs 15 SC) | Not defined | 3 positions (prone, left/right lateral) for 1–2 h every 4 h | Category not standardized | Change in PaO2/ FiO2 ratio at 72 h after admission | No difference between 2 groups (p=0.077) |
| Pierucci et al.23 2021 | Bari, Italy | Observational prospective single-center study | 11 March to 30 April 2020 | 32 COVID-19 patients with PaO2/FiO2 >150 (16 PP vs 16 SC) | Not defined | PP for as long as tolerated | Non-severe and Severe COVID-19 | Feasibility and effects of prolonged PP | After 72 h, 62.5% of PP patients improved oxygenation [PaO2/FiO2: from 194.6 (42.1) to 304.7 (79.3.2), p <0.001] |
| Rosén et al.25 2021 | Helsinki, Sweden | Prospective multicenter open label parallel arm randomized clinical superiority trial | 7 October 2020 to 7 February 2021 with 30-day follow-up till 9 March 2021 | 75 COVID-19 patients (36 PP protocol vs 39 control group) | HFNO, NIV | PP for at least 16 h/day | Critical COVID-19 P/F ≤150 mmHg for more than 1h using HFNO or NIV | Effect of PP protocol in need for ETI | Longer prone in PP vs control group 9.0 h per day (IQR: 4.4–10.6) vs 3.4 h (IQR: 1.8–8.4) (p=0.014), but there was no difference in ETI |
| Sryma et al.26 2021 | Delhi, India | Prospective interventional study | Not defined | 45 COVID-19 patients (33 PP vs 15 SC) | LFNO, HFNO, NIV | PP ≥2 h/session, total PP ≥8 h per day | Severe COVID-19 SpO2 <94% FiO2=21% PaCO2 <45 mmHg | Effect of PP in need for ETI and oxygenation | PP showed improvement in the mean (SD) ROX index [10.7 (3.8) vs 6.7 (2.6), p<0.001]. The need for ETI was higher in the control group (33.3% vs 6.7%, p=0.02) |
| Fralick et al.32 2022 | Canada USA | Unblinded pragmatic randomized clinical trial | May 2020 to May 2021 | 248 patients (126 PP vs 122 SC) | Not defined | PP ≥2 h/session for 4 times day for ≥7 days | Severe and Critical COVID-19 | Composite of in-hospital death, mechanical ventilation (intubation or BPAP) or worsening RF (FiO2 >60% for >24 h) | Same incidence in both groups (OR= 0.92; 95% CI : 0.44–1.92) |
| Perez-Nieto et al.38 2022 | Mexico Ecuador | Retrospective multicenter observational study | 1 May to 12 June 2020 | 827 COVID-19 patients (505 PP vs 322 SC) | Not defined | PP ≥2 h | Severe COVID-19 SpO2 <94% FiO2=21% | Successful orotracheal intubation for invasive mechanical ventilation | PP protective factor for orotracheal intubation (OR=0.35, 95 % Cl: 0.24–0.52, p <0.0002) |
| Ibarra-Estrada et al.33 2022 | Guadalajara, Mexico | Multicenter randomized controlled trial | 2 May 2020 to 26 January 2021 | 414 COVID-19 patients (216 PP vs 198 SC) | HFNO | PP ≥1 h/day | Severe and Critical COVID-19 except for hemodynamically unstable | Intubation rate within 28 days of enrollment | Significantly lower intubation incidence in PP group (RR=0.70; 95 % CI: 0.54–0.90, p =0.006) |
| Altinay et al.28 2022 | Istanbul, Türkiye | Retrospective observational cohort study | 15 March to 15 June 2020 | 48 COVID-19 patients (25 PP vs 23 SC) | NRBM | PP 12–18 h/day | Severe COVID-19 P/F <300 mmHg using NRBM | Differences in PaO2/FiO2 ratio, length of ICU stay and ventilator-free days, mortality and intubation rate in PP vs SC | Lower mortality and intubation incidence in the intervention group (p=0.020, p=0.001), NS difference in other outcomes |
| Musso et al.36 2022 | Turin, Italy | Controlled non randomized trial | 16 December 2020 to 30 May 2021 follow-up till 30 June 2021 | 243 COVID-19 patients under NIV (81 PP vs 162 non-PP) | NIV | PP ≥8 h/one session/day | Severe and Critical COVID-19 except for hemodynamically unstable | Occurrence of NIV failure within 28 days of enrollment, defined as intubation of death | Significantly lower incidence of NIV failure in PP group (p<0.001) |
| Koike et al.35 2022 | Sagamihara, Japan | Retrospective cohort study | 1 October 2020 to 31 March 2021 | 58 COVID-19 patients (27 PP vs 31 SC) | Not defined | PP ≥30 min at least twice/day | Severe and Critical COVID-19 | Effects of PP on the improvement of oxygenation over 3 weeks | PP for patients with FiO2 ≥0.4 was associated with the improvement of short-term SpO2/FiO2 reduction and ROX index and was significantly associated with a lower rate of tracheal intubation (p=0.003) |
| Jha et al.34 2022 | Cambridge, UK | Prospective single-center study | 3 September 2020 to 23 February 2021 | 25 COVID-19 patients and 10 healthy volunteers under hypoxic challenge | LFNO | Cycle of position changes: supine for 15 min, lateral for 15 min, prone for ≥30 min | Non-severe COVID-19 | Change in peripheral oxygenation in PP vs SP | Increase in SpO2 in PP vs SP (difference +1.62%, p=0.003) within 10 min of proning. Increase in subjective discomfort (p=0.003) in PP, with no difference in breathlessness |
| Esperatti et al.30 2022 | Argentina | Prospective multicenter cohort study | June 2020 to January 2021 | 335 COVID-19 patients (187 PP >6 h vs 148 SC) treated with HFNC | HFNO | PP ≥6 h per day | Critical COVID-19 P/F <200 mmHg after receiving 4 h of HFNO | Effect of PP on the risk of ETI and in-hospital mortality | The OR (95% CI) for ETI in the PP group was 0.36 (0.2–0.7), with a progressive reduction in OR as the exposure increased. The AOR (95% CI) for hospital mortality in the PP group was 0.47 (0.19–1.31). PP ≥8 h/d resulted in reduction in OR [0.37 (0.17–0.8)] |
| Fazzini et al.31 2022 | London, UK | Prospective single-center cohort study | 1 March to 30 April 2020 | 46 COVID-19 patients (12 <1 h vs 34 >1 h) | LFNO, HFNO CPAP | PP for as long as tolerated | Severe and Critical COVID-19 | Outcomes of PP vs SC | Oxygenation improvement in PP: P/F ratio (pre, 115 ± 43 mmHg vs post, 148 ± 70 mmHg, p=0.025) and S/F ratio (pre, 141 ± 37 vs post, 188 ± 49, p<0.001), lower RR (pre, 34 ± 7 vs post, 25 ± 7 breaths per min, p<0.001), lower WOB (pre, 43 vs post, 16) and improvements in reported shortness of breath after PP (pre, 45 vs post, 19; p<0.001). PP >1 h had lower ICU admissions (PP ≤1h, 83% vs PP > 1 h, 41%, p=0.011), required less invasive ventilation (PP ≤1 h, 83% vs PP >1 h, 29%, p=0.001) and had shorter median ICU length of stay (LOS) [PP ≤1 h, 13 (5–26) vs PP >1 h, 5 (3–18) days, p=0.016] |
| Tonelli et al.40 2022 | Italy | Retrospective multicenter observational cohort study | 1 March to 1 June 2020 | 114 COVID-19 patients (38 PP vs 76 SC) | Not defined | PP ≥3 h/day, 1–4 sessions/day | Severe and Critical COVID-19 P/F <300 mmHg or SpO2 ≤93% breathing room air RR ≥30 breaths/min | Clinical benefit of PP vs SC of patients with non-invasive respiratory support | Greater effect of PP compared to SC on ETI rate after adjustment for confounders (HR=0.59; 95% CI: 0.3–0.94, p=0.03). PP showed greater significant benefit for those on HFNC (HR= 0.34 ; 95% CI: 0.12–0.84, p =0.04) |
| Qian et al.39 2022 | USA | Non-randomized controlled trial | 13 May to 11 December 2020 | 501 COVID-19 patients with hypoxemia | LFNO, HFNO, NIV | PP ≥3 h for 4 times/day | Severe and Critical COVID-19 | Outcomes of PP vs SC | On day 5 the Bayesian posterior probability of PP group having worse outcomes was 0.998 (posterior median AOR=1.63; 95% credibility interval CrI: 1.16–2.31). On days 14 and 28, the posterior probabilities of harm were 0.874 (AOR= 1.29; 95% CrI: 0.84–1.99) and 0.673 (AOR=1.12; 95% CrI: 0.67–1.86), respectively |
| Othman et al.37 2022 | Damanhour City, Egypt | Randomized controlled trial | 20 February to 20 April 2021 | 82 COVID-19 patients (41 PP vs 41 SC) with PaO2/ FiO2 ratio ≤150 mmHg | NRBM, CPAP | PP ≥1 h/day | Severe and Critical COVID-19 P/F ≤150 mm/Hg RR ≥30 breaths/min | Effects of awake PP on oxygenation and physiological outcomes in non-intubated patients | PP showed improvements in SpO2, PaO2/FiO2, ROX index, PaO2, and SaO2, at the three study time points (p<0 .001, 0.007; p<0.001, 0.011 ; and p<0.001, respectively) |
All the studies included patients with confirmed COVID-19 disease either with positive molecular swab test (RT-PCR) of nasopharyngeal or oropharyngeal sample (28 studies) and/or compatible imaging findings in combination with symptoms indicating infection with SARS-CoV-2 (8 studies, 3 studies, respectively).
Patients with acute hypoxemic respiratory failure (AHRF) were included in 25 studies, with 5 of them setting a condition of PaO2/FiO2 <300 mmHg, another 2 of PaO2/FiO2 >150 mmHg and 2 of them with a condition of PaO2/FiO2 <150 mmHg. Ten studies analyzed 0–50 patients, 10 analyzed 50–100 patients, 9 analyzed 100–500 patients, and 3 analyzed over 500 patients.
In the majority of the studies included in our review, the method of oxygenation was not defined with several types being used, more specifically low-flow nasal cannula (LFNC), simple face mask (SFM), non-rebreather mask (NRBM), high-flow nasal cannula (HFNC), and non-invasive mechanical ventilation either with continuous or bilevel positive airway pressure (NIV). Four studies analyzed patients on HFNC15,20,30,33, 2 studies used NIV16,36, 3 studies used LFNO21,22,34, while Altinay et al.28 included only patients under NRBM. Ates et al.14 subsumed patients with mild COVID-19 with oxygen saturation over 94% therefore not in need of supplementary oxygen therapy.
The duration and initiation time of prone positioning
In 6 studies the duration of PP was determined in daily hours, sessions per day, and total days. In the Musso et al.36 study, the median daily hours of PP were 12.2 (10.1–13.8) with a median of 2 sessions per day (1.3) and total days of PP 6 (5.8) in a period of 28 days. The median PP hours in Koike et al.35 study was 3 (2–3) and the number of practice days of PP therapy was a median of 13 days (7–16). Liu et al.22 noted 12.6 daily hours of PP in both early and late PP groups of patients and a total time of PP of 14.3 days. Patients in Rosen et al. study25 were proned for 9 (4.4–10.6) hours daily with 4.2 (1.7–5.7) days in total. Jayakumar et al.17 defined the adherence to protocol as >6 hours of PP daily which was among 13 (43%) patients of PP group, 4 patients tolerated PP for 5–6 hours, 5 patients for 4–5 hours, 4 patients for 1–4 hours and 2 patients for less than an hour, while 2 patients did not comply with PP. The maximum duration was 2 hours per session. The centers that participated in Ehrmann et al.15 study recorded a range of median duration of PP from 1.6 to 8.6 hours per day.
The initiation time of PP was not determined in most of the studies. Barker et al.29, Jha et al.34, Fazzini et al.31 and Vianello et al.29 assigned all of the enrolled patients to undergo prone position. Those who could not tolerate PP or were contraindicated to prone, formed the control groups. In the study of Sryma et al.26, patients were proned if they had a P/F ratio <100 mmHg using NIV or HFNO, or altered mental status. Pierucci et al.23 started prone positioning after achieving an SpO2 >96% using supplemental oxygen in patients. In the Koike et al.35 study, prone positioning was initiated when the FiO2 reached ≥40%. Ates et al.14 used six positions (prone, left/right lateral decubitus, left/right swimmer’s, and supine). They determined the two positions with the best oxygen saturation by measuring SpO2 after 5 min in each one and afterwards patients were instructed to maintain those two positions. Patients in the Musso et al.36 study were proned 24 h after admission. Lastly, Kharat et al.21 instructed patients to self-prone and report their PP duration in a diary.
We collected and recorded the following outcomes: 1) mortality rate (WHO ordinal scale, ISARIC mortality score); 2) intubation rate; 3) ventilator-free days; 4) oxygenation parameters (ROX index, SpO2/FiO2 or PaO2/FiO2 ratio, ABGs); 5) length of hospital or ICU stay; 6) upgrade or weaning in oxygen therapy; 7) patients’ vitals and use of vasopressors to stabilize arterial blood pressure; and 8) prone positioning adverse events.
Mortality
Mortality rate was examined in 21 studies. Twelve studies showed no difference between prone and supine patients, while the rest (9 studies) noted a statistically significant decrease in mortality rate in prone patients. Specifically, Musso et al.36 recorded 36% mortality in the control group (162 patients) versus 12% in PP (81 patients) (p<0.001), and Altinay et al.28 found 16% in the control group (23 patients) versus 9% in PP (25 patients) (p=0.02). In the studies of Ates et al.14 and Jagan et al.10, none of the patients in prone position died compared to the control group (mortality rate 24.6% out of 65 patients, p<0.004; and 8.5% out of 47 patients, p<0.001; respectively). Kaur et al.20 showed significantly higher mortality in late APP (APP initiated >24 h of starting HFNC therapy) group (45% of 33 patients) versus early APP (APP initiated within 24 h of starting HFNC therapy) (26% of 92 patients) (p=0.039). In Esperatti et al.30 and Perez-Nieto et al.33 studies, the adjusted OR of mortality decreased in PP group (0.38 and 0.40, respectively). Four studies examined the WHO ordinal scale and 1 study the ISARIC mortality score. Padrão et al.12, Koike et al.35 and Rosen et al.25 noted no significant differences in WHO ordinal scale, while Qian et al.39 observed a worse outcome rank in the intervention group from day 2 to 5 (p=0.03). Barker et al.29 proved lower ISARIC mortality score in prone position (p=0.04).
Intubation rate
Ten out of 18 studies showed a statistically significant decrease in intubation rate. Koike et al.35 recorded an intubation rate of 7% in PP group (2 out of 27 patients) vs 42% in SC group (13 out of 42 patients) (p=0.003). Endotracheal intubation was needed in 4 patients (8%) in prone position and in 12 patients (28%) who failed in PP in the Vianello et al.27 study (p=0.014). Sryma et al.26 also noted higher rates of intubation and mechanical ventilation in the control group (33.3%; 5/15 patients) vs prone group (6.7%; 2/30 patients) with p=0.02. In the study of Esperatti et al.30 23% of the PP group (44/187 patients) and 53% of the standard care group (79/148 patients) were intubated (p<0.0001). The rate of intubation in Musso et al.36 was 10% of 81 patients in the intervention group and 32% of 162 patients in the control group (p=0.0012). In the Ibarra-Estrada et al.15 study, 25% of the PP group (29/117 patients) and 41% of the non-PP group (128/313 patients) were intubated (p=0.004). Jagan et al.10 showed as well a lower intubation rate in prone-positioned patients (10% vs 27.7%, p=0.031). According to Perez-Nieto et al.33, 24.8% (77/505) and 39.5% (123/322) of patients were intubated in prone and supine group, respectively (p<0.0001). In the study of Altinay et al.28, 32% of the patients required intubation in the prone position group (8/25 patients) and 82.6% in the supine position group (19/23 patients) with p=0.001. Jouffroy et al.19 reported that at day 10, 40% (16/40) of the PP group and 71% (241/339) of the non-PP group were intubated (p<0.01).
In 3 studies the statistical significance of the difference in intubation rate between the intervention and the control group was not determined15,18,32.
Ventilator-free days
Ventilator-free days were examined in 4 studies and none showed a statistically significant difference between prone and supine patients. Specifically, in the Padrão et al.12 study, ventilator-free days were 8 (2–12) and 6 (0–11) in prone (57 patients) and supine (109 patients) positioned patients, respectively (p=0.4). In Johnson et al.18, the patients in PP were off the ventilator for 24.3 (18.8–29.7) days while in the standard care group for 27 (24.8–29.2) days (p=0.332). Rosen et al.25 recorded 30 (11–30) days without mechanical ventilation in both groups (p=0.69). In contrast, Altinay et al.28 recorded 3.5 (3.0–6.5) days for PP group (25 patients) and 2 (2–3) days for non-PP group (23 patients) (p=0.004).
Oxygenation parameters: SpO2/FiO2 or PaO2/FiO2 ratio, ROX index and ABGs
Oxygenation parameters and ABGs were assessed in 18 studies; 13 studies examined the SpO2/FiO2 or PaO2/FiO2 ratio, with only 3 of them17,18,29 not finding a statistically significant difference between the intervention and the control group. Respiratory rate oxygenation (ROX) index (combination of peripheral oxygen saturation to the fraction of inspired oxygen and RR [SpO2/FiO2]/RR) was evaluated in 5 studies, all of them showing a significant increase in the intervention group11,13,26,35,37.
Another parameter that was assessed in the included studies were ABGs and/or SpO2. In the Othman et al.37 study a significant increase in SpO2 (5.85%, p<0.001), PaO2 (22.59%, p=0.011) and in SaO2 (5.26%, p<0.001) was noted after proning, but without significant difference in pH and PaCO2 (p=0.94 and p=0.83, respectively). In the Zang et al. study13, SpO2 increased from 91.09 ± 1.54% to 95.30 ± 1.72% (p<0.01) after 10 min, 95.48 ± 1.73% after 30 min (p<0.01), but no significant difference after 30 min compared with 10 min (p=0.58). Jouffroy et al.19 showed no difference in SpO2 (92% to 93%, p=0.34) and PaO2 (59 to 62 mmHg, p=0.08) after PP; however, PaCO2 slightly improved (35 to 38 mmHg, p=0.005). Jha et al.34 reported that a lower SpO2 value at admission was predictive of greater improvement in SpO2 with proning (p=0.003) and smaller improvement for older patients (p=0.013). Changes in pH, PaO2, PaCO2 and SpO2 in Altinay et al.28 were 0 (p=0.002), 16 mmHg (p<0.001), -1 mmHg (p=0.007) and 5% (p=0.016), respectively, for proned patients. Finally, Liu et al.22 noted no significant difference in pH and PaCO2 after 24 h (p=0.86 and p=0.40, respectively).
Length of hospital or ICU stay
Duration of hospital stay was assessed in 7 studies, ICU stay in 5 studies, and both parameters in 5 studies. Vianello et al.27 patients were hospitalized for a median time of 17 (6–46) days in PP and 21 (7–75) days in supine position (p<0.001). The median hospital days for PP group were 12.2 and for control group 23.2 in Liu et al.22 (p=0). In the Ates et al. study14, median length of hospital stay was 5 (2–16) vs 2 (3–20) days in PP vs SC group (p<0.001). Less days of hospitalization in proned patients or equal days to standard care group, but not statistically significant, were recorded in 3 studies11,15,18,35.
Altinay et al.28 recorded a median ICU stay of 5 (4–11) vs 8 (4–12) days in the PP vs SC group (Cohen’s d=0.3). Hashemian et al.16 estimated an ICU stay of 8.6 ± 3 days for PP group (NIV + PP) and 14.4 ± 3.9 days for SC group (NIV), with p=0.046. However, in Jayakumar et al.17 and in Barker et al.29, patients in the intervention group were hospitalized in ICU for more days comparing to the control.
Three out of 5 studies showed the beneficial effect of prone position in both hospital and ICU length of stay. Rosen et al.25 recorded a median hospital stay of 16 (11–22) days for the PP group and 18 (11–30) days for the SC (p=0.44), and median ICU stay of 5 (4–13) days for PP and 11 (3–22) for SC (p=0.25). Median hospital and ICU length of stay for PP and SC groups were 12 (7–20) and 9 (6–14) days (p=0.0012) in the Esperatti et al.30 study. In Tonelli et al.40, median hospital stay was 20 (3–41) for PP and 24 (3–45) days for SC (p=0.03), and ICU stay duration of 10 (3–21) for PP and 15 (3–26) days for SC (p=0.02).
Upgrade or weaning in oxygen therapy
In the Prud’homme et al.24 study, 25 (52.1%) patients in SC group (48 patients) and 15 (31.2%) patients in PP group (48 patients) needed upgrade in oxygenation (p=0.038). Vianello et al.27 escalated the respiratory support in 7 (16%) patients in SC group (43 patients) and 2 (4%) in PP group (50 patients) (p=0.047).
Two studies recorded a not statistically significant decrease in supplemental oxygen in proned patients17,21, while Sryma et al.26 noted no difference in time to resolution of hypoxia between the intervention and control group.
Patients’ vitals and use of vasopressors to stabilize arterial blood pressure
Liu et al.22 recorded a decrease in both respiratory and heart rate equal to 3.62 breaths/min and 2.51 beats/min (p=0.005 and p=0.71, respectively). Respiratory rate was assessed in the Ibarra-Estrada et al.15 study with a decrease from 25 to 22 breaths/min after the first PP session (p<0.001). The same parameter was examined in Fazzini et al. study31 where patients had lower respiratory rate after proning (pre, 34 ± 7 vs post, 25 ± 7 breaths/min; p<0.001). After 12 h of PP respiratory rate was significantly different in the intervention group compared to controls in the Sryma et al.26 study (23.8 ± 3.4 breaths/min among cases vs 27.5 ± 4.6 among controls, p=0.004).
Two studies showed an improvement of patients’ vitals (blood pressure, respiratory rate) in prone position, but not statistically significant13,19.
The need for vasopressor use in order to stabilize blood pressure was greater, but not statistically significant, in controls rather than proned patients in the Rosen et al.25 study (44% controls vs 37% PP, p=0.57). In contrast, in the Padrão et al. study12, more patients in PP were administered vasoactive drugs (47% PP vs 39% controls, p=0.32).
Prone positioning adverse events
The reported adverse events in the prone groups were accidental removal of peripheral intravenous lines, back/musculoskeletal pain (limiting prone positioning)12, pressure sores, nausea and vomiting, cardiac arrest within 30 days25, and general discomfort21,35. Musso et al.36 observed that there was no statistically significant difference between the prone and supine group regarding the previously presented adverse events and additional ones which were barotrauma, pneumomediastinum, subcutaneous emphysema, nasal skin ulceration due to nasal cannula, facial edema, thoraco-abdominal wall hematoma, and venous thrombosis. In 2 studies no adverse events occurred11,17.
DISCUSSION
As was previously stated, the aim of the current review was to summarize the evidence of the effect of prone positioning in patients with COVID-19 pneumonia, not on invasive mechanical ventilation, based on published literature.
The most interesting finding of the current review is that most studies showed a beneficial effect of prone positioning in hypoxemia in non-intubated COVID-19 patients, although the intubation rate and mortality varied among the studies.
The benefit of prone positioning on oxygenation in intubated patients with ARDS is well documented41,42 and there is enough evidence that this benefit is sustained also in ARDS due to SARS-CoV-2 infection43. Although prone positioning was not extensively utilized in non-intubated patients before the COVID-19 outbreak, it was a widely used intervention from the beginning of the pandemic due to disease pathophysiology, but also due to the urgent necessity of finding non-invasive therapeutic interventions as a result of the large influx of patients in ICUs worldwide44,45. Before the COVID-19 outbreak, however, a number of studies regarding awake prone position in ARDS patients were published, therefore offering the rationale for the wide use of prone position during the COVID-19 pandemic46,47.
Despite the evidence of beneficial effect of prone position in non-intubated patients with COVID-19, the results of the studies should be carefully evaluated, since a large heterogeneity is detected among researched populations and study designs alike.
There are notable differences among the studies regarding ventilation and oxygenation strategies in non-intubated COVID-19 patients. As previously mentioned, several types of oxygenation and ventilation have been utilized, while the exact type of respiratory support is not defined in the vast majority of the included studies.
According to the results of recent meta-analyses, there are insufficient data to determine differences in mortality reduction between patients who were treated with HFNC or NIV in prone position48,49. In comparison with LFNC, ventilatory support with HFNC or NIV in ICU settings appears to reduce intubation rates; however, these results may reflect differences in disease severity49. Specifically, a reduced need for intubation was shown among patients who received advanced respiratory support (HFNO or NIV) at enrollment (RR=0.83; 95% CI: 0.71–0.97) and in ICU settings (RR=0.83; 95% CI: 0.71–0.97), but not in patients receiving conventional oxygen therapy (RR=0.87; 95% CI: 0.45–1.69) or in non-ICU settings (RR=0.88; 95% CI: 0.44–1.76)49.
An additional factor that varies among the included studies is the prone positioning technique. Whereas the included patients are not sedated, patient cooperation for prone positioning and patient compliance to maintain position are prerequisites for successful intervention. It is interesting that in all studies not showing improvement in oxygenation and/or mortality12,18,21,32,34,39, the patients were verbally instructed to assume and maintain prone position as long as possible, hence the prone position tolerance was poor. Nursing-directed protocols might increase adherence, leading to possible different results18. Several strategies including light sedation have been proposed in order to achieve adherence for long prone position sessions50,51. An important issue is whether the effect of better oxygenation in prone position in patients with COVID-19 is indeed associated with a reduced intubation rate, even in an ICU setting, where the compliance and monitoring are better than in the ward. The study of Barker et al.29 failed to show such a benefit. Nevertheless, in a recent meta-analysis, treatment in ICU setting seemed to be advantageous52.
Another contributing factor that affects prone position outcomes is the exposure time. Prolonged prone position is known to decrease mortality in patients with severe ARDS and also in mechanically ventilated patients with ARDS due to COVID-1953. Similar results were found in our review. The hours in prone position among the studies vary and were not included in the statistical analysis in the majority of the aforementioned studies. Mostly due to patient discomfort, the duration of prone position sessions in most of the studies is relatively small in comparison with invasively ventilated patients, and lack of adherence may be an indicator of disease severity27. Whereas prone position seems to have a time-dependent effect, the optimal exposure time for non-intubated patients is yet to be established.
An important point is that patients with severe COVID-19 disease seem to benefit more from the prone position compared to those with less severe disease. In a recent meta-analysis, of the 1172 patients in the APP group, 281 were intubated, while 329 of the 1122 patients in the control group were intubated. Dividing patients into 2 subgroups (defined as 1: PaO2/FiO2 ≤200 mmHg, and 2: SpO2/FiO2 >200 mmHg), showed that patients with PaO2/FiO2 ≤200 mmHg had a lower intubation rate when compared with the control group (four trials, RR=0.80; 95% CI: 0.71–0.90). Intubation rate in patients with less severe disease (subgroup 2) was decreased although this finding was not statistically significant (four trials, RR=0.93, 95% CI: 0.40–2.19). Regarding mortality rate, the meta-analysis showed no difference between the intervention and the control group (RR=0.93; 95% CI: 0.77–1.11). Although, statistically significant decrease in survival was observed in patients in the APP group with severe disease, defined as PaO2/FiO2 <150 mmHg, highlighting the need for further research in order to establish the association between mortality and prone position54.
Limitations
This review has several limitations. There is a wide heterogeneity regarding patient populations, oxygenation and ventilation methods, disease severity and outcomes, thus the comparison of the results was not feasible for all of the studies. Furthermore, the included studies were conducted during different phases of COVID-19 pandemic and the evolution of other therapeutic interventions was not taken into consideration.
CONCLUSIONS
Prone positioning seems to be an effective intervention for non-intubated COVID-19 patients. Due to the lack of comprehensive protocols, large scale randomized control studies with carefully selected population and thoroughly described interventions should be conducted to confirm the aforementioned effect not only in patients with COVID-19 but also with other causes of pneumonia.