INTRODUCTION
Prostate cancer constitutes an extremely common malignancy in middle-aged men between 45 and 60 years and is the leading cause of cancer-associated morbidity and mortality in the male population of Western societies1,2. It is reported that each year, 190000 new prostate cancer cases arise worldwide, with approximately 80000 deaths1,3. Prostate cancer typically metastasizes to the bones and the regional lymph nodes with a recognizable pattern of spread 4,5. Nevertheless, a few cases of atypical extra nodal metastases of prostate cancer are reported in the literature4,5. Pleural metastasis has been reported only 4 times in the literature, as a peculiar metastasis of prostate cancer, presented as solitary pleural thickening and without clinical manifestations4-7.
Here, we present a rare case of pleural metastasis from prostate cancer, presented as massive pleural effusion with shortness of breath, in a 91-year-old patient without comorbidities and no prior diagnosis of prostate cancer. This case report describes the patient’s management and aims to highlight the key-role of pneumonologists’ clinical awareness, for the prompt diagnosis of this peculiar clinical condition. To the best of our knowledge, this is the first case of pleural metastasis from prostate cancer diagnosed with EBUS-TBNA. The current manuscript adheres to SCARE criteria.
CASE PRESENTATION
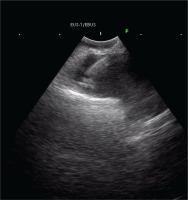
A 91-year-old Caucasian male, non-smoker, without occupational exposure to inhaling substances, was admitted to our department in January 2024, complaining about shortness of breath for the past week. The patient’s history was unremarkable. His vital signs were as follows: blood pressure: 130/77 mmHg, heart rate: 82 beats/min, SpO2 89%, and a body temperature 37.2oC. No clubbing was evident, and physical examination did not reveal any palpable cervical lymph nodes. Clinical examination revealed reduced breath sounds absence in the right hemithorax. Subsequent pleural Ultra Sound and CXR verified large right pleural effusion.
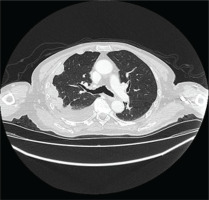
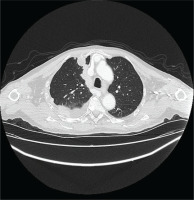
The patient underwent HRCT, that indicated patchy infiltrates in the right lung pleural effusion with adjacent pleural and mediastinal pleural thickening, leading to the provisional diagnosis of pleural malignancy or pleural infection from Mycobacterium tuberculosis (Figures 1 and 2). The patient underwent diagnostic thoracocentesis that was suggestive of lymphocytic exudative pleural effusion. Herein, a chest tube drainage was placed, draining in total 5000 mL. Blood tests revealed increased inflammatory markers (CRP 105 mg/L and mild neutrophilia). The patient was immediately administrated with IV levofloxacin, and he was admitted for further investigation and treatment.
Upon admission, the patient underwent blood tests for: troponins, CK-MB, antinuclear antibodies, rheumatic factor, native-DNA antibody and anti-extractable nuclear antigen analyses, viral infections (such as Sars-CoV-2, HIV, HBV, HCV, CMV, EBV), and blood cultures collected during fever peaks for infectious diseases (leptospirosis, borreliosis, brucellosis, rickettsiosis). All the laboratory results were negative. Tuberculin skin testing (TST), blood, and urine sample culture tests were also negative. Tumor markers’ analysis revealed PSA 135 ng/mL and D-dimers were 505 μg/L. Serum calcium levels and phosphatase alkaline were within normal values.
Four days after the initial thoracocentesis, the patient underwent chemical pleurodesis and two days after the chest tube was finally removed. A bronchoscopy with EBUS-TBNA was performed. The bronchial tree anatomy was normal, and biopsies were taken with EBUS-TBNA by a right paratracheal hypodense lesion of the mediastinal pleura (Figure 3).
The pathological analysis of the biopsies showed histologically neoplastic cells arranged in stripes, nests and irregular glandular formations were seen in a hemorrhagic background. Cells had of a medium-sized, were elongated, and nuclei with variably prominent nucleoli and moderate amount of eosinophilic cytoplasm. Stratification was present.
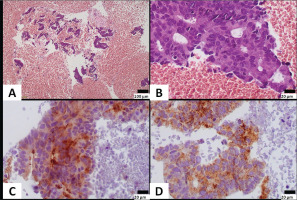
Immunohistochemically, neoplastic cells expressed CK8/18, PSA and AMACR, and were negative for napsin, TTF1, ERG, WT1, calretinin and CK20. Rare cells (<5%) expressed CK7. The pathology report was consistent with adenocarcinoma of prostatic primary, with features of ductal adenocarcinoma indicating pleural metastasis from undiagnosed prostate cancer (Figures 4A–4D).
Figure 4
A) Neoplastic cells are seen in a hemorrhagic background; B) Cells are arranged in irregular granular formations, show stratification and have elongated nuclei with variably prominent nucleoli; C) Neoplastic cells express PSA; and D) AMACR (A: original magnification ×100; B–D: original magnification ×400)
The patient was referred to the oncology department where he was scheduled for further prostate biopsy. Biopsy verified the diagnosis of clinically advanced prostate cancer. CT scan of head and abdomen and bone did not reveal any prostate cancer metastases. Bone scan and PET-CT were not performed during his hospitalization due to the request of the patient to perform these on an outpatient basis. The patient was discharged from the pneumonology department afebrile, hemodynamically stable, respiratory sufficient and clinically improved.
DISCUSSION
Prostate cancer is it documented as one of the most common malignancies in male population worldwide, leading to approximately 190000 new prostate cancer cases each year, and almost 80000 deaths1-3. Despite PSA testing as population screening test, many cases of prostate cancer are diagnosed at a mature stage of the disease, leading to failure of treatment and increased mortality rate among patients1,8.
Prostate cancer typically metastasizes to the bones and the regional lymph nodes, including pelvic and abdominal retroperitoneal lymph nodes, with a recognizable pattern of spread4. Nevertheless, very few cases of atypical extranodal metastases from prostate cancer are reported in the literature, including metastasis to: kidneys, lungs, liver, spleen pancreas, breasts, peritoneum, parotid gland, soft tissues4, the adrenal glands4,6, and the pleura 4-7.
According to the literature, little attention has been given on documenting the radiological and clinical features of the peculiar sites of metastasis from prostate cancer4. Pleural metastasis has been reported only 4 times in the literature, as a peculiar metastasis of prostate cancer, presented as solitary pleural thickening5 and without clinical manifestations5-7. It is also documented that pleural metastasis may manifest as circumferential pleural thickening, mediastinal pleural involvement, nodularity or irregularity of the pleural contour, and infiltration of the chest wall5. In our case, the patient presented with shortness of breath and pleural effusion, and chest CT revealed pleural thickening along with pleural effusion.
A retrospective study of 620 prostate cancer patients by Vinjamoori et al.4 documented that both PSA levels and Gleason core did not correlate with any specific distribution of disease, metastasis potentiality and pattern, or tumor histological grade4. However, it was reported that pleural effusions were encountered more frequently in prostate cancer patients with concurrent osseous metastasis, which included the spine in all cases4,9,10. This phenomenon may be explained by the fact that the venous system represents the conduit of metastasis in such cases11. Nevertheless, our patient presented a pleural metastasis from prostate cancer without obvious osseous metastasis or brain metastasis. Nonetheless, PET-CT or bone scan were not performed during hospitalization, due to the patient’s request. Hence, the exact pathway responsible for metastasis to the pleura remains unclear5.
As for the applicability and safety of pleural biopsy through EBUS-TBNA, only a few case reports exist in the literature12,13. Nevertheless, it is a reasonable alternative to other methods of biopsy of the pleura, with good safety profile and reasonable diagnostic yield14.
Finally, as in all cases with stage IV prostate cancer, patients with pleural metastasis from prostate cancer, should be administrated with androgen ablation along with surgical or pharmacological castration1,10. It is documented that first-generation antiandrogens such as flutamide and bicalutamide may be beneficial. However, in stage IV, castration resistance, due to genomic mutations in the androgen receptor, may occur and hinder treatment and the prognosis15.
CONCLUSIONS
Despite its rarity, physicians should be aware of such rare cases of a typical metastasis from prostate cancer. The presence of pleural metastasis should be investigated in cases of male patients with elevated PSA and/or a known history of prostate cancer. Finally, although typically asymptomatic, pleural metastasis from prostate cancer may present with shortness of breath or signs and symptoms of respiratory infection. When encountered, it is extremely important to investigate the potentiality of osseous metastasis as well, to ameliorate patient’s management.