INTRODUCTION
Drug overdose is a serious public health problem requiring increasing usage of intensive care resources globally1. In the United States, the number of patients with drug overdose requiring admission to the intensive care unit (ICU) significantly increased by 34% between 2009 and 20152. Around one in four of those patients presented with acute hypoxemic respiratory failure (AHRF)2. Potential mechanisms of drug-related AHRF may include (but not limited to): 1) impairment of consciousness leading to aspiration and subsequent pneumonia, and 2) direct insult of the lung parenchyma leading to pulmonary capillary leak and subsequent non-cardiogenic pulmonary oedema. The latter appears after abuse of opioids (such as heroin), cocaine, and amphetamines3. No matter what the underlying mechanism is, AHRF may be present in about 95% of fatal drug overdose cases4,5, which substantially increased during the pandemic of the new coronavirus disease6.
Although drug overdose is a recognized risk factor of AHRF, accounting for almost 2% of AHRF cases according to the large multicenter epidemiological LUNG SAFE study7, a direct comparison between drug overdose and other risk factors of AHRF seems lacking in the literature. It is not, therefore, well known whether AHRF associated with drug overdose has distinct features compared to AHRF associated with other risk factors. We hypothesized that drug-overdose associated AHRF may be associated with lower mortality compared to non-drug-overdose associated AHRF, probably due to confounders. For this reason, we endeavoured to compare the clinical characteristics and outcomes of patients with AHRF associated or not with drug overdose.
METHODS
Study design and patient population
We performed a secondary analysis of individual patient-level data from the LOTUS FRUIT study8. The LOTUS FRUIT study was a multicenter, prospective, observational cohort study conducted by the Prevention and Early Treatment of Acute Lung Injury (PETAL) Network and enrolled consecutive adult patients with acute respiratory failure who were admitted to ICU and received invasive mechanical ventilation8. Up to 100 patients per participating hospital were enrolled during a 30-day period between 1 July and 1 October 2016, and were followed until hospital discharge or day 28. Patients receiving chronic invasive mechanical ventilation through a tracheostomy, patients admitted to the ICU after elective surgery, those presenting to the hospital after more than a day of invasive mechanical ventilation, or those extubated before being transferred to the ICU, were excluded.
For the present secondary analysis, we included patients with AHRF following a two-step process. Firstly, given that AHRF necessarily encompasses acute respiratory distress syndrome (ARDS, a severe form of AHRF)9, we included in our analysis all patients determined by the LOTUS FRUIT investigators to have ARDS on the day of intubation (defined as the partial pressure of arterial oxygen to fraction of inspired oxygen ratio PaO2:FiO2 ≤300, not fully explained by cardiac failure or fluid overload, and bilateral infiltrates not fully explained by mass, collapse, or effusion on chest radiography as reviewed by site investigator)8,10. Secondly, we included in our analysis patients who did not meet all of the abovementioned criteria of ARDS, but they were intubated due to acute hypoxemia (defined as oxygen saturation SpO2 <90% or PaO2 <60 mmHg)8.
We categorized patients with AHRF into the two compared groups of the present secondary analysis, namely, the ‘drug-overdose associated AHRF’ group (when drug overdose was mentioned as either the sole risk factor or one of the risk factors associated with AHRF in a given patient) and the ‘non-drug-overdose associated AHRF’ group (when drug overdose was not mentioned among the risk factors associated with AHRF in a given patient). The latter group of ‘non-drug-overdose associated AHRF’ also included cases when no risk factor of AHRF was identifiable (256 patients, 20% of the included population)11,12. As previously13,14, the Biologic Specimen and Data Repository Information Coordinating Center of the National Heart, Lung, and Blood Institute provided us with the requested data in a de-identified form after submission of a prospective protocol. The protocol was approved by the Institutional Review Board (protocol number 398/9-11-2022), which also waived the need for informed consent (non-human subjects research).
Outcomes
The primary outcome of the present analysis was 28-day mortality, with patients discharged from the hospital with unassisted breathing prior to 28 days considered to be alive at 28 days. Secondary outcomes were differences in ventilator-free days, ICU-free days and prevalence of rapidly improving AHRF between compared groups through day 28 following intubation. As previously15,16, ventilator-free days were defined as the number of days from the end of the last period of assisted breathing up to day 28. Hospitalized patients who died before day 28 were considered to have zero ventilator-free days. ICU-free days were defined as the number of days that the patient was alive and not in the ICU. Rapidly improving AHRF was defined as extubation or having a PaO2 to the fraction of inspired oxygen (FiO2) ratio greater than 300 on the first day following intubation17-19.
Statistical analysis
We present continuous variables as median (interquartile range) and compare them using the Mann-Whitney U test. We present categorical variables as frequencies and percentages and compare them using the chi-squared or Fisher’s exact test, as appropriate. We assess the association between drug overdose and 28-day mortality (primary outcome) using a Cox proportional hazards regression analysis, both unadjusted and adjusted. The adjusted analysis takes into consideration age and the concurrent (i.e. along with drug overdose) presence of sepsis or shock as risk factors of AHRF. To construct the Cox proportional hazards regression model, we used all available information on outcomes (such as mortality) and the included variables. There were no missing data on outcomes, except from ICU-free days (15.9% missing values).
Also, we conducted a mediation analysis20 by considering drug-overdose associated AHRF as the independent predictor of 28-day mortality and rapidly improving AHRF as the potential mediator. We examined whether variations in the mediator could explain the differential outcomes of patients with drug-overdose associated AHRF as opposed to patients with non-drug-overdose associated AHRF21. For the mediation analysis, we applied logistic regression of the generalized linear models to fit the binary mediator and outcome, and we utilized the nonparametric bootstrap for variance estimation. All p values were two-sided, and we considered statistical significance at an α level of 0.05. We conducted all statistical analyses using SPSS software version 28.0 (SPSS, Inc., Chicago, IL) and R software version 4.2.1, with the R Package for Causal Mediation Analysis for the mediation analysis (R Foundation for Statistical Computing).
RESULTS
Baseline characteristics
Supplementary file Figure 1 presents the flow diagram of patients included in the LOTUS FRUIT study. The present secondary analysis included 1280 patients with AHRF, of whom 684 (53.4%) met all definition criteria of ARDS, while the remaining 596 patients (46.6%) only met the acute hypoxemia criterion (Supplementary file Figure 1). Of the 1280 patients with AHRF, 48 (3.8%) had drug-overdose associated AHRF. Table 1 depicts the baseline characteristics of patients with AHRF in each of the compared groups. Patients with drug-overdose associated AHRF were younger (42.0 vs 60.0 years, p<0.001), and were less likely to have sepsis (12.5% vs 29.1%, p=0.012) or shock (6.3% vs 18.1%, p=0.035) as risk factors of AHRF than patients with non-drug-overdose associated AHRF. Compared groups did not differ substantially in terms of organ failures and respiratory variables on the day of intubation. This was also the case for the first day following intubation, i.e. there was no difference between compared groups in terms of PaO2:FiO2, tidal volume per predicted body weight, plateau pressure, and respiratory rate (Table 1).
Table 1
Baseline characteristics of patients with AHRF in each of the compared groups during a 30-day period between 1 July and 1 October 2016 (N=1280)
| Characteristics | All (N=1280) n (%) | Drug overdose (N=48)a n (%) | Non-drug overdose (N=1232) n (%) | p |
|---|---|---|---|---|
| Age (years), median (IQR) | 60.0 (47.0–69.0) | 42.0 (31.0–49.8) | 60.0 (48.0–69.0) | <0.001 |
| Females | 522 (40.8) | 16 (33.3) | 506 (41.1) | 0.284 |
| Race | 0.514 | |||
| White | 773 (66.8) | 34 (75.6) | 739 (66.5) | |
| Black | 234 (20.2) | 6 (13.3) | 228 (20.5) | |
| Hispanic or Latino | 105 (9.1) | 3 (6.7) | 102 (9.2) | |
| Asian | 32 (2.8) | 1 (2.2) | 31 (2.8) | |
| American Indian or Alaskan Native | 13 (1.1) | 1 (2.2) | 12 (1.1) | |
| Risk factors of AHRF | ||||
| Pneumonia | 311 (24.3) | 13 (27.1) | 298 (24.2) | 0.646 |
| Aspiration | 182 (14.2) | 10 (20.8) | 172 (14.0) | 0.181 |
| Sepsis | 365 (28.5) | 6 (12.5) | 359 (29.1) | 0.012 |
| Trauma | 125 (9.8) | 1 (2.1) | 124 (10.1) | 0.080 |
| Shock | 226 (17.7) | 3 (6.3) | 223 (18.1) | 0.035 |
| Otherb | 111 (8.7) | 0 (0.0) | 111 (9.0) | 0.018 |
| On the day of intubation | ||||
| Renal failure | 312 (29.0) | 6 (12.8) | 306 (29.8) | 0.012 |
| Liver failure | 175 (16.3) | 4 (8.5) | 171 (16.6) | 0.140 |
| Coagulation failure | 257 (23.9) | 7 (14.9) | 250 (24.3) | 0.138 |
| Median (IQR) | Median (IQR) | Median (IQR) | ||
| PaO2:FiO2 | 146.5 (87.8–216.0) | 157.0 (82.9–250.0) | 146.3 (87.9–216.0) | 0.518 |
| Tidal volume per predicted body weight | 7.0 (6.1–8.0) | 6.5 (6.1–7.9) | 7.0 (6.1–8.0) | 0.251 |
| Plateau pressure | 21.0 (17.0–25.0) | 18.0 (15.0–23.5) | 21.0 (17.0–26.0) | 0.051 |
| Respiratory rate | 20.0 (16.0–25.0) | 20.0 (15.0–31.5) | 20.0 (16.0–25.0) | 0.666 |
| On the first day following intubation | ||||
| PaO2:FiO2 among intubated patientsc | 169.0 (115.0–237.0) | 165.0 (111.8–211.4) | 170.0 (115.0–237.5) | 0.667 |
| Tidal volume per predicted body weight | 6.7 (6.0–7.8) | 6.3 (6.1–7.6) | 6.7 (6.0–7.8) | 0.472 |
| Plateau pressure | 20.0 (17.0–24.0) | 19.0 (16.0–22.0) | 21.0 (17.0–24.0) | 0.369 |
| Respiratory rate | 20.0 (16.0–26.0) | 21.5 (16.5–29.3) | 20.0 (16.0–25.0) | 0.336 |
IQR: interquartile range. AHRF: acute hypoxemic respiratory failure. PaO2:FiO2: partial pressure of arterial oxygen to fraction of inspired oxygen ratio.
a Twenty-three (47.9%) out of the 48 patients with drug-overdose associated AHRF had at least one more risk factor of AHRF (other than drug overdose).
Outcomes of patients
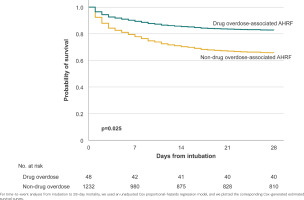
Table 2 depicts the outcomes of patients with AHRF in each of the compared groups. Patients with drug-overdose associated AHRF had lower unadjusted mortality (16.7% vs 34.4%, p=0.011) than patients with non-drug-overdose associated AHRF. In an unadjusted Cox proportional hazards-regression analysis, patients with drug-overdose associated AHRF had lower mortality by day 28 following intubation than patients with non-drug-overdose associated AHRF (hazard ratio, HR=0.450; 95% CI: 0.223–0.905, p=0.025) (Table 3). Figure 1 depicts the corresponding survival curves for each group.
Table 2
Outcomes of patients with AHRF in each of the compared groups during a 30-day period between 1 July and 1 October 2016 (N=1280)
| Outcome | All (N=1280) | Drug overdose (N=48) | Non-drug overdose (N=1232) | p |
|---|---|---|---|---|
| 28-day mortality, n (%) | 432 (33.8) | 8 (16.7) | 424 (34.4) | 0.011 |
| Ventilator-free daysa, median (IQR) | 19.0 (0.0–26.0) | 24.5 (17.3–27.0) | 18.0 (0.0–26.0) | <0.001 |
| ICU-free daysb, median (IQR) | 16.0 (0.0–24.0) | 24.0 (17.0–27.0) | 15.0 (0.0–24.0) | <0.001 |
| Rapidly improvingc AHRF, n (%) | 326 (25.5) | 24 (50.0) | 302 (24.5) | <0.001 |
a Ventilator-free days were defined as the number of days from the end of the last period of assisted breathing up to day 28. Hospitalized patients who died before day 28 were considered to have zero ventilator-free days.
c Rapidly improving AHRF was defined as extubation or having a partial pressure of arterial oxygen to a fraction of inspired oxygen ratio greater than 300 on the first day following intubation. Extubation on the first day following intubation took place for 22 (45.8%) of the 48 patients with drug-overdose associated AHRF and 201 (16.3%) of the 1232 patients with non-drug-overdose associated AHRF.
Table 3
Cox proportional-hazards regression analyses to isolate the contribution of drug overdose, age and the concurrent (i.e. along with drug overdose) presence of sepsis or shock as risk factors of AHRF (independent variables) to the 28-day mortality (dependent variable)
Figure 1
Survival curves of patients with drug-overdose associated acute hypoxemic respiratory failure (AHRF) and non-drug-overdose associated AHRF during a 30-day period between 1 July and 1 October 2016 (N=1280)
With regard to secondary outcomes (Table 2), patients with drug-overdose associated AHRF, as opposed to non-drug-overdose associated AHRF, had more ventilator-free days (24.5 vs 18.0 days, p<0.001), more ICU-free days (24.0 vs 15.0 days, p<0.001) and were more likely to develop rapidly improving AHRF (50.0% vs 24.5%, p<0.001).
Table 3 depicts a Cox proportional-hazards regression analysis to isolate the contribution of drug overdose, age and the concurrent (i.e. along with drug overdose) presence of sepsis or shock as risk factors of AHRF (independent variables) to the 28-day mortality (dependent variable). In the adjusted analysis, drug overdose was no longer associated with lower mortality among patients with AHRF (adjusted hazards ratio, AHR=0.584; 95% CI: 0.288–1.185, p=0.136).
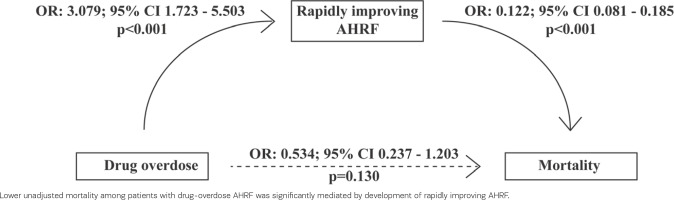
Given that patients with drug-overdose associated AHRF were more likely to develop rapidly improving AHRF than patients with non-drug-overdose associated AHRF, we conducted a mediation analysis by considering drug-overdose associated AHRF as the independent predictor of 28-day mortality, and rapidly improving AHRF as the potential mediator. The mediation analysis is depicted in Figure 2. Lower unadjusted mortality among patients with drug-overdose AHRF was significantly mediated by the development of rapidly improving AHRF (p<0.001 for the average causal mediation effect).
DISCUSSION
By incorporating data from 1280 patients with AHRF enrolled in the LOTUS FRUIT prospective observational study8, the present secondary analysis showed that patients with drug-overdose associated AHRF were younger and more likely to develop rapidly improving AHRF than those with non-drug-overdose associated AHRF. Also, patients with drug-overdose associated AHRF had lower unadjusted mortality compared to patients with non-drug-overdose associated AHRF. However, after adjustment, drug overdose was no longer associated with lower mortality and in a causal mediation analysis, lower unadjusted mortality among patients with drug-overdose AHRF was found to be significantly mediated by the development of rapidly improving AHRF.
Despite considerable recent evidence on the epidemiology of critically ill patients with drug overdose2,22-26, there might still be a lack of studies directly comparing AHRF associated with drug overdose as opposed to AHRF associated with other risk factors. This was revealed in a relevant systematic review which we performed in order to identify observational studies reporting on clinical characteristics and mortality of patients with drug-overdose associated AHRF. The protocol of the systematic review was registered with PROSPERO (CRD42022363770) and is available online27. Eligible studies reported that patients with drug-overdose associated AHRF were young and had short duration of mechanical ventilation (median duration up to 5.0 days)28-30. Some, but not all, patients with drug-overdose associated AHRF included in those studies also met the criteria of ARDS28-30. Moreover, previous studies reported that almost half of patients with drug-overdose associated AHRF got extubated the day after intubation; i.e. they had rapidly improved AHRF28,31. The above clinical features (young age and rapidly improving AHRF) were confirmed in our analysis. The originality of our analysis lies in that, contrary to the abovementioned studies22-26,28-31, it directly compared patients with drug-overdose AHRF and patients with non-drug-overdose associated AHRF.
We found that patients with drug-overdose associated AHRF had lower (16.7% vs 34.4%) unadjusted mortality than patients with non-drug-overdose associated AHRF. This finding was in line with a recent study of ICU patients hospitalized with severe pneumonia, who reported that drug abuse was associated with decreased in-hospital mortality (OR=0.46; 95% CI 0.39–0.53) compared to no substance abuse32. Such findings (lower unadjusted mortality of patients with AHRF associated with drug overdose compared to other risk factors) should not lead to the misinterpretation that drug-overdose associated AHRF may be inconsequential. Indeed, after adjusting for confounders such as age, we found that mortality associated with drug overdose was comparable with mortality associated with other risk factors among patients with AHRF. Moreover, lower unadjusted mortality among patients with drug-overdose AHRF was significantly mediated by the development of rapidly improving AHRF. Taken together, the above findings may provide valuable insights into the association between drug overdose and mortality among patients with AHRF.
Limitations
The present analysis has limitations. Although there were available high-quality data from 1280 patients with AHRF, conducted by the PETAL Network, drug-overdose associated AHRF was present in 48 patients (i.e. the sample size of our analysis was not large). Even so, this analysis allowed us to perform the first study, to our knowledge, that directly compares AHRF associated with drug overdose and AHRF associated with other risk factors. Also, information was lacking regarding the type of drug used by the enrolled patients, which is an important limitation given that different drugs may have different clinical respiratory pictures and severity. However, this is not unusual for studies on ARDS7. Besides, given that the LOTUS FRUIT study took place in North America in 20168, one may assume that most drug-overdose cases were due to opioids.
CONCLUSIONS
This secondary analysis of the LOTUS FRUIT study showed that patients with drug-overdose associated AHRF were younger and had lower unadjusted mortality than patients with non-drug-overdose associated AHRF. However, this difference in mortality seemed to be due to confounders, such as age, and to be mediated by the development of rapidly improving AHRF. These results may provide insights into the association between drug overdose and mortality among patients with AHRF.