INTRODUCTION
Coronavirus Disease 2019 (COVID-19) is a severe acute respiratory infection that first emerged in 2019 in Wuhan, China1. With 343 million cases recorded in October 2020, COVID-19 has become a global pandemic2. The clinical manifestation of COVID-19 infection tends to be broad, ranging from mild upper respiratory tract disease to severe lung infection with respiratory failure, and often death3. The symptoms are also frequently associated with the extrapulmonary organs, and the kidneys are vulnerable organs to this infection4.
One of the most severe complications of COVID-19 is acute kidney injury (AKI)5. A large number of acute kidney injury cases were found among patients with confirmed COVID-19. As stated in ‘Kidney Disease: Improving Global Outcomes’ guidelines, the incidence rate of AKI in hospitalized adults in China was 11.6%, but for inpatients in the intensive care unit (ICU), the incident rate increases to 50%6. However, those conditions could be worsened by other medical conditions accompanying the COVID-19 patients. Several medical conditions, such as hypertension and diabetes, have similar traits in affecting COVID-19 and AKI patients7.
In COVID-19 patients, the incidence of AKI becomes a crucial prognostic factor. However, no sources specifically discussed the risk factors of AKI in COVID-19 patients. Diabetes and hypertension are several comorbidities of COVID-19 and the risk factors of AKI, which are easily observed and well documented in COVID-19 patients worldwide. As a result, this meta-analysis is intended to explore the correlation between hypertension and diabetes as the risk factors of AKI in COVID-19 patients.
METHODS
Study design
This study aimed to determine the association between diabetes and hypertension as the risk factors for AKI in COVID-19 patients. Therefore, we calculated the combined odds ratio (OR) and a 95% confidence interval (95% CI) using a random or fixed-effect model. To ensure the quality of our study, we created a meta-analysis using PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist8.
Literature search strategy
The search string used in our present study was adapted from Medical Subject Heading (MeSH) comprising: [Acute Kidney Injury] AND [(SARS-CoV-2) OR (COVID-19)] AND [(Hypertension) OR (Diabetes Mellitus)]. The search strategy was conducted in PubMed, Google Scholar, and Medline, up to 21 March 2022. Additionally, we searched the reference lists of pertinent literature for potentially relevant articles in English. We only included articles with the larger sample sizes, if several studies with similar study data were found. Three independent authors carried out the search strategy to meet the required standards (SS, IRS, BP).
Study eligibility
The articles included in our study met the following criteria: 1) articles with case-control, cross-sectional, and cohort designs; 2) articles identifying AKI progression risk variables in COVID-19 patients, and the prevalence of acute kidney injury in groups (with the 2012 Kidney Disease Improving Global Outcomes guidelines used to diagnose AKI during hospitalization); and 3) presenting the number of patients with hypertension or diabetes mellitus for estimating OR and 95% CI. On the other hand, articles were excluded if they: 1) had irrelevant titles or abstracts; 2) were reviews and case reports; 3) had insufficient and/or unspecific data; 4) were unpublished studies; 5) were low quality; and 6) did not define the diagnostic criteria for AKI.
Data extraction
The data extracted from each study were: 1) type of literature study; 2) total patients with AKI; 3) total patients without AKI; 4) total patients with DM for each group; and 5) total patients with HT for each group. Three investigators (SS, IRS, BP) worked together to collect the data. If there were differences, a discussion was carried out to reach an agreement.
Quality assessment
Three independent authors (SS, IRS, BP) evaluated the quality of the articles in our analysis using the Newcastle-Ottawa Scale (NOS), based on three factors consisting of patient selection (4 points), comparability (2 points), and exposure determination (3 points). The total score ranged from 0 (worst) to 9 (best). The study was considered good if the score was 7, moderate if the score was 5, and bad if the score was <59. We consulted a senior author if there were differences between the three independent authors (JKF, BD, AG).
Statistical analysis
We analyzed the risk factors of AKI in COVID-19 patients by calculating using the Z test, and the estimation effect was determined by calculating OR and 95% CI. Potential publication bias and heterogeneity were assessed before assessing the relationship and impact estimates. To validate publication bias, we performed the Egger test and a funnel plot. Publication bias was described as a p<0.05. Additionally, the Q test was used to assess heterogeneity. If p<0.10, the random effect model was applied, if the p≥0.10, the fixed-effect model was applied. The statistical program R (R Core team, Vienna, Austria) version 4.0.4 was used for all our analyses10.
RESULTS
Eligible studies
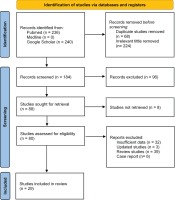
Our literature search strategy collected 476 potentially relevant studies published from October 2020 to March 2022. From the identified studies, 68 duplicates and 224 studies with irrelevant tittle were excluded. The remaining 184 studies were screened; 96 studies were excluded due to irrelevance, and 8 studies were unavailable to be reviewed. As a result, there were 80 studies reviewed, but only 29 studies met our inclusion criteria11-39, and 51 studies were excluded. The reasons for the exclusion included insufficient data (32 studies), updated data (3 studies), review paper (39 studies), and case report (6 studies). The flowchart of the study selection process is shown in Figure 1, while the baseline characteristics of the included studies are provided in Table 1.
Table 1
Baseline characteristics of included studies
| Author Year | Study type | Country | City | Hospital | COVID-19 severity | N | Sample size | Risk factors | NOS | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AKI+ | AKI- | Age (years), mean (SD) | DM n | HT n | Obesity n | ||||||||
| Alfano et al.11 2020 | Cohort retrospective | Italy | Modena | University Hospital of Modena | All cases | 307 | 69 | 238 | 65.2 (14.01) | 54 | 138 | 28 | 9 |
| Bowe et al.12 2021 | Cohort retrospective | USA | St Louis | VA St. Louis Health Care System | All cases | 5216 | 1655 | 3561 | 69 (11.12) | 2537 | 3985 | - | 8 |
| Casas-Aparicio et al.13 2021 | Cohort retrospective | Mexico | Mexico City | Instituto Nacional de Enfermedades Respiratorias | Severe cases | 99 | 58 | 41 | 52.9 (13.2) | 27 | 30 | 56 | 9 |
| Chan et al.14 2021 | Cohort retrospective | USA | Multi-center | Mount Sinai Health system | All cases | 3993 | 1835 | 2158 | 66 (16.31) | 1019 | 1527 | - | 9 |
| Cheng et al.15 2020 | Cohort retrospective | China | Wuhan | Tongji Hospital | All cases | 1392 | 99 | 1293 | 65.33 (20.78) | 241 | 499 | - | 9 |
| Costa et al.16 2021 | Cohort retrospective | Brazil | Rio de Janeiro | Hospital Unimed-Rio | All cases | 102 | 57 | 45 | 66.35 (15.75) | 32 | 55 | - | 8 |
| Doher et al.17 2020 | Cohort retrospective | Brazil | Sao Paulo | Hospital Israelita Albert Einstein (HIAE) | Severe cases | 201 | 101 | 100 | 65.33 (11.94) | 64 | 98 | 8 | |
| Fominskiy et al.18 2020 | Cohort retrospective | Italy | Milan | San Raffaele scientific institute | Severe cases | 96 | 72 | 24 | 57.85 (8.00) | 16 | 42 | 8 | |
| Hamilton et al.19 2020 | Cohort retrospective | UK | Manchester | Multi-center | All cases | 1032 | 210 | 822 | 70 (20.04) | 335 | - | - | 9 |
| Hansrivijit et al.20 2021 | Cohort retrospective | USA | Multi-center | Multi-center | All cases | 283 | 115 | 168 | 64.1 (15.9) | 108 | 189 | 132 | 9 |
| Hectors et al.21 2021 | Cohort retrospective | USA | New York | Weill Cornell Medicine | All cases | 45 | 16 | 29 | 62 (55.90) | 13 | 26 | - | 8 |
| Jewell et al.22 2021 | Cohort retrospective | UK | London | King's College Hospital | All cases | 1248 | 487 | 761 | 69 (17.1) | 406 | 681 | - | 9 |
| Joseph et al.23 2020 | Cohort retrospective | France | Paris | Saint Louis Hospital | All cases | 100 | 81 | 19 | 59.66 (10.53) | 30 | 56 | 21 | 8 |
| Lee et al.24 2020 | Cohort retrospective | USA | New York | Multi-center | All cases | 1002 | 294 | 708 | 65 (17.07) | 378 | 597 | 184 | 8 |
| Li et al.25 2021 | Cohort retrospective | China | Shanghai | Shanghai Public Health Clinical center | All cases | 1249 | 91 | 1158 | 37.66 (17.07) | 63 | 142 | 353 | 8 |
| Mohamed et al.26 2020 | Cohort retrospective | USA | New Orleans | Ochsner Medical center | All cases | 575 | 161 | 414 | 63 (12.5) | 281 | 242 | - | 8 |
| Ng et al.27 2021 | Cohort retrospective | USA | New York | Multicenter | All cases | 9019 | 3216 | 5803 | 65.92 (2.8) | 3469 | 5730 | 3323 | 9 |
| Nimkar et al.28 2020 | Case series | USA | New York | Montefiore New Rochelle Hospital | All cases | 327 | 179 | 148 | 70.66 (17.12) | 139 | 209 | - | 9 |
| Öztürk et al.29 2021 | Cohort retrospective | Turkey | Multi-center | Multicenter | All cases | 621 | 202 | 419 | 60 (19.3) | 172 | 332 | - | 9 |
| Phillips et al.30 2021 | Cohort retrospective | UK | Southampton | University Hospital Southampton | All cases | 632 | 216 | 416 | - | 142 | 321 | 179 | 9 |
| Procaccini et al.31 2021 | Case control | Spain | Madrid | Hospital Universitario Infanta Leonor | All cases | 1096 | 548 | 548 | 75.09 (13.7) | 328 | 701 | 56 | 9 |
| Russo et al.32 2021 | Cohort retrospective | Italy | Genoa | Hospital Policlinico San Martino | All cases | 777 | 176 | 601 | 70 (16.0) | 16 | 49 | - | 9 |
| Sang et al.33 2020 | Cohort retrospective | China | Wuhan | Multicenter | All cases | 210 | 92 | 118 | 63 (11.0) | 44 | 98 | - | 9 |
| Tan et al.34 2020 | Cohort retrospective | China | Shenzhen | Third People’s Hospital of Shenzhen | All cases | 377 | 40 | 337 | 45.2 (17.7) | 19 | 65 | - | 8 |
| Wang et al.35 2020 | Case control | China | Wuhan | Multicenter | All cases | 275 | 136 | 139 | 69.33 (11.17) | 62 | 150 | - | 9 |
| Xiao et al.36 2021 | Cohort retrospective | China | Wuhan | Hankou Hospital | All cases | 287 | 55 | 232 | 61 (14.2) | 45 | 87 | - | 9 |
| Xu et al.37 2020 | Cohort retrospective | China | Multi-center | Jin Yin Tan Hospital | All cases | 671 | 263 | 408 | 64.66 (12.62) | 131 | 287 | - | 9 |
| Zamoner et al.38 2021 | Cohort retrospective | Brazil | Sao Paulo | University Hospital Sao Paulo | All cases | 101 | 51 | 50 | 55 (54.4) | 34 | 22 | 22 | 8 |
| Zhang et al.39 2020 | Cohort retrospective | China | Wuhan | Multi-center | All cases | 282 | 123 | 159 | 69 (13.41) | 61 | 146 | - | 9 |
Data synthesis
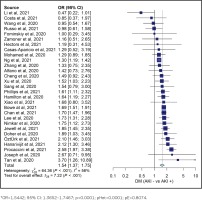
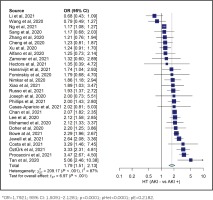
Our study analysis shows that diabetes increased the risk of AKI in COVID-19 patients (OR=1.5442; 95% CI: 1.3652–1.7467, p<0.0001, pHet=0.0001, pE=0.8074). Our study also indicated an increased risk of AKI in COVID-19 patients with hypertension (OR=1.7921; 95% CI: 1.5091–2.1281, p<0.0001, pHet<0.0001, pE=0.2182). These results were obtained from 10698 AKI patients with COVID-19 and 20917 controls from 29 studies. The summary of the estimated effect of the risk factors AKI in COVID-19 is provided in Table 2.
Table 2
Summary of the risk factors of COVID-19 induced AKI
Heterogeneity among studies and publication bias
Overall, evidence of heterogeneity was found in all groups. Therefore, the included studies in each group were calculated using a random-effect model. However, the Egger test was not significant in the hypertension and diabetes groups, indicating no publication bias. The Egger test was found significant in the obesity groups, indicating publication bias. Detailed information of the heterogeneity and Egger tests is summarized in Table 2, and in Figures 2 and 3.
DISCUSSION
Our meta-analysis results emphasized that comorbidities, such as hypertension and diabetes, may give an early warning to physicians about AKI progression in patients with COVID-19. These two comorbidities are known to be associated with COVID-19 severity40, but their relationship with AKI progression was still to be discussed. The previous meta-analysis conducted by Kaminska et al.41 exclusively discussed the outcome of COVID-19 patients with diabetes, and the result showed that AKI is one of the associated complications. Further, a previous meta-analysis by Fabrizi et al.42 observed the outcome of AKI in COVID-19 patients, and their study also showed the association between hypertension and AKI in COVID-19 patients. Therefore, our meta-analysis supports the result of the previous meta-analyses in which diabetes and hypertension increase the risk of AKI in COVID-19 patients.
The condition accompanying COVID-19 patients has a pivotal role when the physician starts prognosing and managing COVID-19. Our studies focused on how the comorbidities affect the progression of COVID-19 into kidney injury. SARS-CoV-2, the virus causing COVID-19, infects the lung cells through the angiotensin-converting enzyme 2 (ACE2) receptor43. The expression of ACE2 receptor is not only observed in the lung cells, but also in the renal cells at the proximal tubular cells and podocytes; thus, making it possible to involve them directly44. Moreover, tubular necrosis resulting from indirect causation is the most common pathogenesis of AKI, as in 60% of COVID-19 patients26. It may occur either from ischemic tubular injury or toxic tubular injury as an impact of various mechanisms, such as hypovolemia, cytokine release, and medication-induced ramifications from the underlying COVID-19 infection45. Of the mechanisms, the gap for explanation may exist to link the comorbidities and the deterioration of renal functions in COVID-19 patients.
The interesting part of SARS-CoV-2 infection is how it combines diabetes and hypertension as two distinct medical conditions that may affect the renal cells in the same way. Diabetes and hypertension interrupt ACE2 expression in patients without COVID-1946. Instead of protecting by reducing the viral entry receptor in podocytes and tubular cells, the high affinity of the SARS-CoV-2 virus to the ACE2 receptor further worsens ACE2 deficiency7,47. This condition leads to the activation of angiotensin II that fails to be converted into inactive peptides by ACE248. Angiotensin II will induce the pro-inflammatory cytokine and cause the inflammatory response to damage the protein, lipid, and DNA in the renal structure, and this condition leads to glomerular filtration rate reduction7. The pro-inflammatory cytokines, such as IL-1, IL-6, and TNF-α, are responsible for this mechanism, and among COVID-19 patients, they are considered to play a key role in ischemic acute kidney injury pathophysiology49.
Our results, thus, stress the complexity of the AKI management in COVID-19 patients with diabetes and hypertension to the clinicians. In COVID-19 patients, the goal of diabetes and hypertension treatments is to manage those conditions and to prevent AKI. Patients with diabetes must be aware of the benefits and risks of sodium-glucose co-transporter-2 (SGLT2) conflicting issues. SGLT2 has an anti-inflammatory effect, but its involvement in kidney secretion function renders the patients prone to AKI due to ketoacidosis50. In addition, long-term diabetic patients have pathological lesions in the nephron, including arteriosclerosis, thickening of the glomerular basement membrane, accumulation of extracellular matrix, and interstitial fibrosis51. Both direct and indirect damages caused by the COVID-19 virus to the nephron worsen acute kidney injury. Therefore, insulin treatment is much favorable, notably in COVID-19 patients with critical conditions52. Moreover, COVID-19 patients with hypertension are facing the safety issue of angiotensin receptor blockers (ARB) and angiotensin-converting enzyme inhibitor (ACEI) medication. A study conducted by Oussalah et al.53 concluded that ARB and ACEI increased the risk of AKI and respiratory failure in 149 COVID-19 patients due to lung and kidney organ-crosstalk. Furthermore, an increase in intraglomerular capillary pressure often occurs in patients with long-standing hypertension. Thus, arterionephrosclerosis is often present54. Decreased renal perfusion associated with hemodynamic disturbances also causes a decrease in glomerular filtration rate55. Hemodynamic disturbances found in COVID-19 patients possibly increase the risk of decreasing perfusion and filtration in hypertensive patients with COVID-19. However, the cornerstone of AKI treatment in patients with and without COVID-19 is fluid management. However, in severe COVID-19 patients, the clinicians need a robust effort to inhibit the AKI progression. At the moment, continuous renal replacement therapy is generally used, but different treatment strategies, such as monoclonal antibody and plasma convalescent therapy, are popular as the last alternative56.
Limitations
Our meta-analysis has some limitations. This study did not include other related comorbidities, such as age and cardiovascular disease, and other risk factors, such as medication, disease severity, the use of mechanical ventilation, and treatment related to COVID-19 or AKI. Although there is a link to AKI progression in COVID-19 patients, only a handful of studies discuss those factors. Additionally, a bigger population is needed to evaluate the true effect of each variable. Further research that includes more high-quality studies are required to provide better evidence.
CONCLUSIONS
Our meta-analysis shows that diabetes and hypertension increase the risk of acute kidney injury (AKI) induced by COVID-19. The reason behind this result is still not well explained, but the inflammation process has a major role in the pathophysiology of AKI progression in COVID-19 patients. However, future studies are needed to reveal the reasons behind this pathophysiology.