INTRODUCTION
The health-related quality of life (HRQoL) of patients with asthma is affected by the presenting symptoms and their limiting effects on their physical, work and social aspects of their everyday life1. Hence, early and effective control of the disease can significantly impact their HRQoL, as assessed through various tools, and, in turn, decrease the use of healthcare resources1,2.
When referring to disease control in asthmatic patients, two aspects should be considered, the repression of symptoms and the reduction of the risk for potentially unfavorable outcomes, which include the incidence of exacerbations, persistent effects on lung function, and adverse events emerging from the treatment itself. In prospect, inadequate symptom control usually results in increased exacerbations and overall a negative effect on the quality of life2. Spirometry and, particularly, persistent airway obstruction are additive parameters for predicting future risk2.
Self-reported outcomes and physician-assessed tools provide a method for harmonizing and quantifying asthmatic patients’ initial state and changes occurring thereafter. The Asthma Control Questionnaire (ACQ) is a 7-item questionnaire that incorporates and evaluates symptoms and the treating guidelines. Certain cut-off scores, ranging from 0.75–1.50 have been proposed to differentiate between ‘well controlled’ and ‘not well controlled’ patients3. Validated translations of the ACQ-7 questionnaire in different languages, including Greek, are available4. The concurrent use of more than one of these assessment tools may not offer practical advantages to the clinician, as the available questionnaires may not exhibit the same correlation to the patient’s symptoms5.
The initial evaluation and monitoring of the patient’s quality of life are often overlooked in daily clinical practice, in favor of time conservation and clinical and laboratory examinations. However, in conditions such as asthma, where the disease can produce debilitating symptoms that affect the person’s health perception and their ability for social activity, the assessment of the quality of life can significantly steer the therapeutic goals required. The Asthma Quality of Life Questionnaire (AQLQ) was developed specifically for asthmatic patients, compared to other generic tools that provide only a general representation6. To address the limited resources and time available in clinical trials, a shorter version, the Mini Asthma Quality of Life Questionnaire (MiniAQLQ), was constructed with 15 instead of 32 self-reported items, which has been validated and translated in the Greek language7-9.
The combined use of ACQ-7 and MiniAQLQ has wide acceptance among clinicians and offers measurable insights into disease control. Long-term follow-up studies comparing these tools to lung function tests have noted a high interpatient variability of the latter without a necessary correlation to the self-assessment presented by patients. These results suggest that clinical indicators alone cannot provide an accurate measurement of the effect of treatment on the patient’s quality of life, which may be obtained instead through validated questionnaires10.
The Feeling of Satisfaction with Inhaler-10 (FSI-10) questionnaire is a self-reported instrument containing 10 questions, each with 5 possible responses on a 5-point Likert scale (5=‘very’, 4=’fairly’, 3=‘somewhat’, 2=‘not very’, 1=‘hardly at all’) with maximum total score 50. It assesses the level of satisfaction of the patients with the inhaler and includes items on ease or difficulty of use, portability, and usability. FSI-10 has been validated and translated into the Greek language11,12.
According to Global Initiative for Asthma (GINA) 2021 guidelines, for patients receiving maintenance inhaled corticosteroid (ICS) treatment with as-needed short-acting beta-agonist (SABA), adding long-acting beta-agonist (LABA) in a combination inhaler provides additional improvements in symptoms and lung function with a reduced risk of exacerbation compared with the same dose of ICS13,14.
Very limited published data of the effectiveness of the fixed-dose combination budesonide/formoterol (BUD/ FORM) administered via the Elpenhaler® device in a real-world setting are available for Greece. The present study, the BOREAS study, aimed to additionally collect information on the patients’ asthma control, quality of life, comorbidities, treatment effectiveness regarding the absolute number of patients’ eosinophils count, safety, and the satisfaction from the use of the Elpenhaler® inhalation device.
METHODS
The study was conducted with a non-interventional design that did not impose any therapeutic restriction or evaluations, in order to capture the typical clinical practice of physicians in Greece. A summary of the study has been published at clinicaltrials.gov (ID: NCT03033758). The study was conducted between February and December 2017 and included hospitals in Athens, Thessaloniki, Chania, Kozani, and Lamia, and private medical offices from a wide geographical area in Greece. In total, 1230 adult asthmatic patients were enrolled from 73 sites. To be enrolled, patients should have been recently prescribed a fixed-dose combination of BUD/FORM via the Elpenhaler® device in doses of 200/6 μg, or 400/12 μg as a maintenance treatment for diagnosed asthma (according to the investigator) and not be concomitantly receiving another combination of ICS and LABA. Exclusion criteria included age <18 years, chronic obstructive pulmonary disease, the use of any ICS/LABA combination, inappropriate use and non-adherence to inhalation treatments. Evaluations were performed at baseline, 3 months, and 6 months, and included ACQ-7, MiniAQLQ, spirometry parameters, and the FSI-10 questionnaire.
The primary objective of the study was the effect of the fixed-dose combination BUD/FORM Elpenhaler® treatment, using a steady daily dose, on the frequency and severity of asthmatic symptoms, as evaluated by the ACQ-7 at 6 months. Secondary objectives were the impact on the QoL as evaluated by the change in the MiniAQLQ total score, lung function evaluation through FEV1 and forced vital capacity (FVC) changes, as well as the incidence of adverse events, disease exacerbations, and hospitalizations. Product satisfaction with the Elpenhaler® device was additionally assessed using the FSI-10 questionnaire at 3 months.
Endpoints also included the evaluation of blood biomarkers, such as the eosinophils count where available, vital signs, and concomitantly used medications and comorbidities, for a possible correlation with the treatment response.
The calculation of the sample size was based on the primary endpoint, namely the change in the ACQ-7 questionnaire from the start of treatment with BUD/FORM Elpenhaler® to 6 months (± 2 weeks). According to the SMARTASIA study, which was conducted on inadequately controlled asthmatic patients, the administration of budesonide formoterol resulted in a statistically significant improvement in the ACQ questionnaire by 0.58 units (SD=0.93 units) after a 12-week treatment period. In addition, according to the study of Kuna et al.15, the administration of budesonide formoterol resulted in a statistically significant improvement in the ACQ questionnaire by at least 0.7 units after a 24-week treatment period. Therefore, based on the aforementioned data, a sample size of 1200 patients was considered to be adequate in order to estimate a change from baseline to 6 months in the ACQ-7 questionnaire of at least 0.7 units, with a 95% confidence level and the power exceeding 90%.
Continuous characteristics are presented in the form of mean±SD values, as well as in the form of median and IQR (interquartile range, between the 25th and 75th percentile). Categorical characteristics are presented in the form of frequencies and percentages. The paired samples t-test was used in order to investigate the change in the examined characteristics between two visits, while the repeated measures analysis of variance (ANOVA) was used in order to investigate the change of the examined characteristics among the three visits (baseline, 3 months, and 6 months).
RESULTS
Overall, 96% (1181/1230) of the patients enrolled successfully completed the visit at 3 months and 94.3% (1160/1230) at 6 months. The mean age was 51.10±16.98 years, with 60.3% (742/1230) of the participants being female and 63.7% (783/1230) never smokers (Table 1).
Table 1
Baseline characteristics of participants (N=1230)
| Characteristics | % |
|---|---|
| Age (years), mean ± SD | 51.1 ± 17.0 |
| BMI (kg/m2), mean ± SD | 28.3 ± 5.6 |
| Sex | |
| Female | 60.3 |
| Male | 39.7 |
| Race | |
| White | 99.6 |
| Black | 0.3 |
| Other | 0.1 |
| Smoking status | |
| Non-smoker | 63.7 |
| Current smoker | 18.2 |
| Former smoker | 18.1 |
| Vaccination | |
| Influenza vaccine | 27.4 |
| Pneumococcus | 20.6 |
| Onset from diagnosis | |
| Previously diagnosed patients | 50.2 |
| Newly diagnosed | 49.8 |
| Previous treatment for asthma | |
| Without previous treatment, % (n) | 41.0 (504) |
| With previous treatment, % (n) | 56.9 (700) |
| SABA | 21.5 |
| ICS+SABA | 14.4 |
| ICS+SAMA | 0.2 |
| ICS | 9.5 |
| ICS+SABA+THEOPHYLLINE | 0.1 |
| LABA | 2.1 |
| ICS+LABA | 1.6 |
| ICS+LABA+SABA | 0.1 |
| LABA+LTRA | 0.1 |
| ICS+LTRA | 0.2 |
| ICS+LTRA+SABA | 0.4 |
| LTRAs | 0.7 |
| LTRA+SABA | 0.9 |
| Other* | 5.1 |
| Missing data | 2.1 |
| Evaluation of asthma control (n=1210) | |
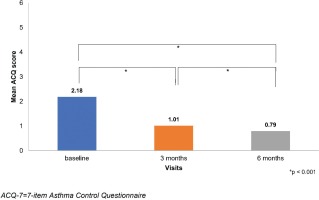
| ACQ-7 score, mean ± SD | 2.18 ± 0.91 |
| Evaluation of QoL (n=1228) | |
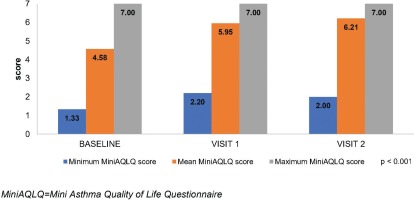
| MiniAQLQ score, mean ± SD | 4.58 ± 1.06 |
* Previous treatments for asthma labelled as ‘Other’ include: anti-IgE, antibiotics, antihistamines, and other various combinations of the treatments included in the table in small percentages. SD: standard deviation. BMI: body mass index. SABA: short-acting betaagonist. ICS: inhaled corticosteroid. SAMA: short-acting muscarinic antagonist. LABA: longacting beta-agonist. LTRA: leukotriene receptor antagonist. ACQ-7: seven-item asthma control questionnaire. QoL: quality of life. MiniAQLQ: mini asthma quality of life questionnaire.
Of the total population, 41.0% (504/1230) of the enrolled patients did not receive any treatment for their asthma before starting the fixed-dose combination BUD/FORM treatment, and 56.9% (700/1230) had a previous treatment (Table 1).
Primary objective
The mean ACQ-7 score was statistically improved between baseline and the visits at 3 and 6 months. The ACQ-7 score decreased from 2.18±0.91 at baseline to 1.01±0.70 at 3 months and 0.79±0.66 at 6 months (Table 1).
Secondary objectives
Similar to the ACQ-7, the MiniAQLQ score significantly increased from 4.58±1.06 (Table 1) to 5.95±0.79 and 6.21±0.74, at the respective time points (Figure 2). A significant, strong, negative, linear correlation between the change at 6 months in ACQ-7 score (-1.4) and MiniAQLQ score (1.6) (rs=0.758, p<0.001) was observed. The FSI-10 tool was used to assess the functionality and ease of use of the Elpenhaler® device, reporting a mean score 44.4±5.0 out of 50.
The most commonly reported comorbidities were diseases of the upper respiratory system (34.7%, 427/1230), followed by cardiovascular diseases (20.0%, 246/1230), metabolic diseases (16.2%, 199/1230), respiratory infections (10.9%, 134/1230), gastroesophageal reflux disease (GERD) (10.6%, 130/1230), psychiatric disorders (5.9%, 73/1230), skin diseases-eczema (2.0%, 25/1230) and obstructive sleep apnea (OSA) syndrome (2.0%, 25/1230).
The mean forced expiratory volume at 1 second (FEV1) also exhibited a gradual improvement with treatment (p<0.0001), from 2.36±0.86 at baseline to 2.58±0.88 and 2.64±0.88 L at 3 and 6 months, respectively.
The incidence of adverse events (AE) was very low, with only 7 patients with AEs, of whom 4 were deemed as having AEs possibly-related with the treatment (hoarseness and oropharyngitis) and 3 other non-related events documented. There were no reported discontinuations of treatment due to AEs. Disease exacerbations were reported in 14 patients, however without any need for hospitalization.
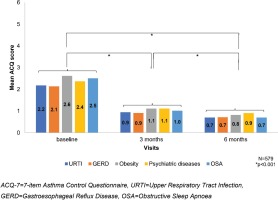
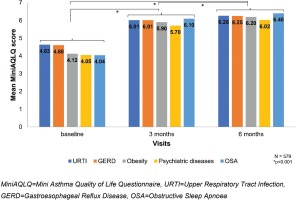
An analogous decrease in the ACQ-7 and increase in the MiniAQLQ (Table 2) score over time was noted when contrasting patients without any comorbidity to those with at least one comorbidity, without identifying any other trends compared to the respective results in the total population. The additional analysis in the subgroup of asthmatic patients (47.1%, 579/1230) with the following five most common comorbidities according to GINA: an upper respiratory tract infection (URTI) (34.7%), GERD (10.6%), obesity (7.8%), psychiatric disorders (5.9%), and OSA (2%), at baseline, the median score of ACQ-7 was 2.2±1.0 (Table 3, Figure 3) and of the MiniAQLQ was 4.5±1.1 (Table 4, Figure 4). After six months of treatment, there was a significant decrease in the ACQ-7 score (p<0.001) and an increase in the MiniAQLQ score (p<0.001).
Table 2
Evaluation of the asthma control via the ACQ-7 scale and MiniAQLQ scale at baseline, 3 months and 6 months among patients with at least one comorbidity and patients without any comorbidity
Table 3
Evaluation of the asthma control via the ACQ-7 scale at baseline, 3 months, and 6 months in relation to the five most common comorbidities according to GINA
Table 4
Evaluation of quality of life via the MiniAQLQ scale at baseline, 3 months and 6 months in relation to the five most common comorbidities according to GINA
A subgroup analysis was performed in 14.6% (180/1230) of patients with an absolute number of eosinophils/ mL in peripheral blood greater than 150. In the subgroup with a mean of 352±202.9 eosinophils/mL in peripheral blood at baseline, the mean ACQ-7 score was 2.29±0.91 (Supplementary file Material 1) and the mean MiniAQLQ score was 4.35±1.07 (Supplementary file Material 2). These are significantly lower values compared with the subgroup of 6.3% (77/1230) patients with an absolute number of eosinophils/mL in peripheral blood ≤150 and a mean of 88±51.1 eosinophils/mL of peripheral blood (ACQ-7 score = 2.10±1.11; MiniAQLQ score = 4.43±1.17). Following six months of treatment, there was a statistically significant decrease in the ACQ-7 score (p<0.001) with a mean of 0.77±0.59, as well as a significant increase in the MiniAQLQ score (p<0.001) with a mean of 6.27±0.66.
Furthermore, asthma control and QoL were assessed in a subgroup of 16.2% patients (199/1230) with metabolic disorders among other comorbidities (i.e. obesity, type 2 diabetes mellitus (T2DM), and dyslipidaemia). At baseline, the mean ACQ-7 and MiniAQLQ scores were 2.45±0.97 and 4.21±1.13, respectively, for the group of patients with metabolic disorders, whereas the mean ACQ-7 and MiniAQLQ scores were 2.13±0.89 and 4.65±1.03, respectively, for the group of patients without metabolic disorders. After 6 months, a significant decrease in the ACQ-7 score (p<0.001), a significant increase in the MiniAQLQ score (p<0.001), and a significant, strong, negative, linear correlation between the change in the ACQ-7 (-1.61) and MiniAQLQ (1.93) score (p<0.001) between visits, were noted for the group of patients with metabolic disorders (Supplementary file Materials 3 and 4). Similar results were also observed in patients without metabolic disorders.
Asthma control and QoL were also assessed in a subgroup of 5.9% patients (73/1230) with psychiatric disorders (i.e. depression, anxiety, and panic disorder). At baseline, the mean ACQ-7 and MiniAQLQ scores were 2.37±1.23 and 4.05±1.31, respectively, in patients with psychiatric disorders and 2.17±0.89 and 4.61±1.03 in patients without psychiatric disorders, respectively. After 6 months, a significant decrease in the ACQ-7 score (p<0.001), a significant increase in the MiniAQLQ score (p<0.001), and a significant, strong, negative, linear correlation between the change in the ACQ-7 (-1.48) and MiniAQLQ (1.95) score (p<0.001) between visits, were noted in patients with psychiatric disorders (Supplementary file Materials 5 and 6). Similar results were also observed in patients without psychiatric disorders.
No other important change resulting from treatment was noted between baseline measurements and the observational visits in the vital signs (blood pressure, cardiac frequency).
DISCUSSION
After six months of treatment with the fixed-dose combination BUD/FORM via the Elpenhaler®, there was a clinically significant improvement in asthma control and QoL of asthmatic patients, as shown using the difference in the ACQ-7 and MiniAQLQ scores, respectively.
Furthermore, a clinically significant improvement in asthma control and QoL were noted in asthmatic patients with five common comorbidities, especially, in those with OSA and obesity, despite the absolute eosinophil number/ mL in peripheral blood. Similar results in the ACQ-7 and MiniAQLQ scores were noted when comparing data from patients with one to four comorbidities and those free from other diseases, to the overall analysis population.
The presence of metabolic or psychiatric disorders followed the statistically significant trends of the total population and did not produce any meaningful correlation with the course of the disease.
The results of large-scale epidemiologic studies in Greece indicate that the prevalence of asthma and asthma-related symptoms is higher than the relevant global metrics16,17. Despite this, real-world studies that aim to elucidate the effect of maintenance treatment with fixed-dose combination BUD/FORM via Elpenhaler® device in this regional pool of asthmatic patients are very scarce.
The BOREAS study aimed to analyze the information captured by self-reported and physician-evaluated tools that is integral to the clinical and functional tests evaluated in asthma. Such data can play a decisive role in the treatment goals set for these patients, especially in those with a stable over-time lung function or an inconsistency between their symptomatology and paraclinical tests. The ACQ-7 and MiniAQLQ were chosen since they have both been translated and validated in the Greek language and are widely accepted in everyday practice. Since this was an observational study, no imposition was set on the choice of treatment, clinical and paraclinical evaluations, or visiting schedule of asthmatic patients, to minimize the limitations on generalizability associated with the rigidly defined evaluations of an interventional trial.
No comparative analyses were planned for the different dosing groups since no significant differences were expected using an individualized treatment approach.
The main finding of the BOREAS study was that asthma control with steady maintenance therapy of a fixed-dose combination BUD/FORM was significantly improved as early as 3 months and continued to do so at 6 months. The rapid therapeutic improvement, as assessed by the ACQ-7, could be anticipated, since other reports have shown this effect to take place as early as 2 weeks after treatment initiation18. The sustainability and further improvement at 6 months without a dose increase in the fixed-dose combination BUD/ FORM regimen has similarly been described in a retrospective analysis of clinical trials, despite that it involved the use of reliever or rescue medications19.
If the suggested judicious cut-off point of 1.5 to identify ‘not well controlled’ patients is used, then the population overall did not meet the criterion for adequate control at the time of enrolment, with the opposite being true for the two follow-up evaluations3. It could be assumed that the significant improvement in the ACQ-7 score could be attributed to: 1) the step-up of treatment since 56.9% (700/1230) were receiving some type of anti-asthmatic treatment including ICS standalone, ICS plus SABA, ICS or SABA as needed, LTRAs, LTRA+SABA, as has been shown in other studies with inadequately controlled patients who switched to a fixed-dose combination BUD/FORM20; and 2) the 41% (504/1230) of patients that were naïve to
treatment. Furthermore, it has been suggested that the future risk of a patient not being regulated by treatment is inversely tied to the extent of their current disease control19,21. Despite their initial disease status of not being adequately controlled, the population of the BOREAS study did achieve asthma control at the end of their observation.
As it was anticipated, the results of the MiniAQLQ correlated with those of the ACQ-7 for symptom control and treatment goal attainment, nonetheless in a statistically significant fashion in both visits. Similar observations on the significant improvement in the quality of life, as measured through the MiniAQLQ, have been published even for short regimens of a fixed-dose combination BUD/FORM of 4, 8, or 12 weeks. A one-year follow-up study in patients with asthma has shown that improvements in the AQLQ were maintained over time in a similar fashion between dosing of 200 and 800 μg of budesonide, with and without formoterol10.
No direct effect on the disease course could be identified from the analyses of the study’s endpoints in subgroups according to the eosinophil count and type of comorbidities. Similar findings came to light from a recent open-label controlled trial in 668 patients either receiving albuterol, budesonide, or budesonide plus formoterol. According to their results, no clear effect to the disease’s exacerbations or ACQ-5 score could be deduced from the age, sex, baseline smoking status, history of exacerbations, baseline SABA use, baseline score on the ACQ-5, FEV1%, and baseline blood eosinophil count22.
Among the patients who participated in the study, a subgroup of 47.1% (579/1230) of patients had at least one of the five most frequently encountered comorbidities that according to GINA guidelines13, are present in patients with asthma (URTI, GERD, psychiatric disorders, obesity, and OSA). These have been commonly reported in other studies, with various possible mechanisms being proposed as the link with asthma, including affected levels of inflammatory mediators, cellular stress, and mechanical anatomic effects23-27. Since multiple chronic conditions tend to occur concurrently, affect the patient’s overall quality of life and exposure to healthcare services, and influence the severity of the disease, the management of asthma patients with comorbidities should be addressed as a whole24,27-29.
Exacerbations were documented in just over 1% (14/1230) of the participants. Despite an annualized rate could not be objectively calculated due to the 6 months follow-up, the projected rate of exacerbations of any severity is in alignment with the range noted from other interventional and observational studies22,30-33. Contrary to the findings of certain randomized trials with high-dose ICS, metanalyses of interventional and observational studies concluded that fewer exacerbations occur with a fixed-dose combination of ICS/LABA than with an ICS alone34,35. Furthermore, the rate at which the improvement occurred was much faster with a fixed-dose combination BUD/FORM versus budesonide monotherapy18.
It has been shown that adding formoterol in patients already using an ICS, such as budesonide, significantly reduces the risk of severe exacerbations and improves lung function, compared to up-titrating the dose of the ICS36.
The analyzed scores of the FSI-10 reflected very high levels of participants’ satisfaction from the use of the Elpenhaler® inhalation device11. Essentially, an improved satisfaction with the inhaler has shown to result in increased adherence and compliance, improved disease control, and therefore reduced healthcare use and associated costs37. Comparative studies between different types of inhalers have shown that switching from one to another might indeed significantly affect the control of asthma38. Therefore, clinicians should be aware not only of the efficacy of the drugs that are planned for administration but also of the effectiveness and compliance rates of the specific inhaling devices that these are contained in. A comparative study of different inhalers available in the Greek market has shown that Elpenhaler® is met with significantly higher satisfaction in most of the domains of the FSI-10 compared to the other devices39.