INTRODUCTION
The first case of coronavirus disease 2019 (COVID-19) was reported in Wuhan city, China, in December 2019, and it rapidly spread across the globe. The World Health Organization (WHO) declared COVID-19 a pandemic on 11 March 2020. While the number of new cases and deaths caused by COVID-19 has recently decreased, as of 5 May 2024, WHO has reported about 775 million confirmed cases and about 7million deaths due to COVID-191.
The multisystem impacts of the disease, including pulmonary, cardiovascular, neurological, and physical effects, have been increasingly reported in patients after recovery from COVID-19 infection. Recent studies suggest that the lung is the major target organ of COVID-192, with alterations in lung function observed in patients after recovery from infection3-9. However, inconsistent results have been reported.
In the first study, conducted in China, lung function was investigated in patients 5–9 weeks after hospital discharge for COVID-19, revealing a restrictive ventilatory defect and impairment of small airway function3. Another study in China observed impaired diffusion capacity and restrictive ventilatory defects in patients 2–6 weeks after the onset of COVID-194. In a study in France reported abnormal lung function in more than half of COVID-19 patients and at the first 30 days after recovery from COVID-19 infection5. Evaluating long-term changes in pulmonary function in patients from Lombardy, Italy, at 6 weeks, 6 months, and 12 months after recovery from COVID-19, Fumagalli et al.6 reported a significant improvement in lung function at 6 and 12 months after hospital discharge. However, a study in Florence, Italy, indicated the persistence of respiratory symptoms and lung function alterations, as indicated by diffusing lung capacity for carbon monoxide (DLCO), at 3–6 months after hospital discharge7. These symptoms tended to normalize or significantly improve at one year after recovery from COVID-19 infection8. Contrasting results were reported by Mogensen et al.9 who found no evidence of mild-to-moderate COVID-19 affecting lung function at six months after recovery in a Swedish study. However, the authors did not report the effects of COVID-19 on lung function in the early period after recovery from infection. Taken together, alterations in lung function might be detectable in the early period after recovery from COVID-19 infection.
Poor sleep quality is frequently observed in recovered patients10,11. Recently, Jackson et al.12 reported that overall poor sleep quality, assessed by the Pittsburgh Sleep Quality Index (PSQI), was associated with impaired pulmonary function in cases at 2–7 months and 10–14 months after hospital discharge for COVID-19. Those authors also found that longer sleep duration and lower sleep efficiency were present in cases after 2–11 months of recovery from COVID-19 when compared with cases after ≥12 months post-infection. These findings suggest that poorer sleep quality might be found in the early period after recovery from COVID-19 infection, possibly related to changes in pulmonary function. However, associations between the components of sleep quality indicated by PSQI scales, particularly sleep duration and sleep efficiency, and pulmonary function have not been demonstrated in the previous studies12.
In Vietnam, the first case of COVID-19 infection was reported on 23 January 2020, and there were about 11.6 million confirmed cases. Of these, there were 43206 deaths and about 10.6 million recovered cases (update on 13 April 2024)13 with the approximate rate of mild-to-moderate illness estimated at 85.2%14. In this study, we evaluated the impacts of mild-to-moderate COVID-19 infection on lung function and sleep quality in young to middle-aged patients during the early recovery period. And then, the relationships between lung function and sleep quality were analyzed to investigate whether the effects of COVID-19 on lung function associate with impaired sleep quality in adults at young and middle age. Our findings contribute to the existing literature on the effects of COVID-19 on lung function and sleep quality, providing valuable insights for the management of patients after recovery from COVID-19 infection not only in Vietnam but also in other countries.
METHODS
The study participants
In Vietnam, all information, including age, sex, location of confirmed cases, deaths, and recovered cases for COVID-19, was reported by the Vietnam Ministry of Health15. In this study, 116 patients were recruited at the 103 Military Hospitals in Hanoi City, Vietnam, from February to May 2022, based on the following criteria: 1) mild-to-moderate COVID-19 infection diagnosis; 2) age range from 18 to 40 years; and 3) No significant medical history. The diagnosis of COVID-19 was determined according to the guidelines of the WHO16 and the Vietnam Ministry of Health17. Mild-to-moderate COVID-19 was determined based on the guidelines of the Vietnam Ministry of Health18. Mild illness was defined as the absence of symptoms or having non-specific symptoms of COVID-19, such as fever, cough, headache, tiredness, with a normal respiratory rate (<20 breaths/min) and peripheral capillary oxygen saturation (SpO2) >96% at room air. Moderate illness included patients who showed tachypnea for age, SpO2 of 93–96% in room air, and lung lesions <50% on chest X-rays. At that time, four patients did not successfully perform the spirometry test, and two patients refused to participate in this study. We also recruited 50 healthy young adults who were not previously diagnosed with COVID-19 but with an age range similar to that of patients with COVID-19. Finally, data of 160 subjects, including 110 recovered cases from mild-to-moderate COVID-19 and 50 healthy young adults, were available for analysis.
Information regarding age, sex, smoking, alcohol consumption, the number of vaccine doses (AstraZeneca, Vero Cell, Pfizer/BioNTech, Moderna) administered against COVID-19, medical history (including obstructive lung diseases, restrictive lung diseases, or other medical conditions), and history of exposure to other risk factors for lung diseases, such as silica or coal dust, was collected from both groups. For the group of patients who recovered from COVID-19, additional data included the date of positive and negative tests for COVID-19, symptoms, and the treatment method during the infection with COVID-19.
Written informed consent was obtained from all participants and all procedures were performed according to a process reviewed and approved by the Vietnamese Military Medical University (No.2091/QD-HVQY, 2022).
Pulmonary function test
After the patients tested negative for COVID-19 for 1 week to 3 months, they underwent an examination of their pulmonary function in the military hospital 103, Hanoi, Vietnam. Pulmonary function was assessed using spirometry (MasterScreen Pneumo; CareFusion, Hoechberg, Germany) to determine forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), FEV1/FVC ratio, forced expiratory flow between 25% and 75% of vital capacity (FEF 25–75%), forced expiratory volume in 3 s (FEV3), average expiratory flow rate between the first 200 mL and 1200 mL of exhaled volume during an FVC maneuver (FEF 200–1200), and peak expiratory flow (PEF). A new disposable mouthpiece was attached to the device. Patients were asked to sit upright and closed their noses with pegs. Deep inspiration was carried out after at least 3 resting tidal respirations followed by forced expiratory maneuver. Each patient performed the test for three times and the best result was recorded. The results obtained by the spirometry test were reviewed and interpreted by two specialists for pulmonary function tests. The measured values are expressed as a percentage of the predicted values using the Global Lung Initiative reference material19. Before the lung function examination, the spirometry system was calibrated to ensure accurate results. Body size indices, including patient weight and height, were measured on the examination day. All spirometry tests were performed according to the guidelines of the Vietnam Ministry of Health20.
Sleep quality assessment
The PSQI was used to assess sleep quality in this study. The reliability and validity of the scale have been previously demonstrated for Vietnamese population21 and it has been used in various countries to evaluate patients after recovery from COVID-19 infection10-12. The scale comprises 19 questions and evaluates sleep quality across seven components, including subjective sleep quality, sleep latency, sleep duration (h), sleep efficiency (hours slept/hours in bed), sleep disturbances, use of sleep medication, and daytime dysfunction22,23. Each component is rated on a scale from 0 (no difficulty) to 3 (severe difficulty). The overall PSQI score is determined by summing up the scores from the seven components, resulting in a range between 0 and 21. The higher scores indicate worse sleep quality and total score ≥5 is considered poor sleep quality22,23.
Statistical analysis
The data analysis was conducted using the SPSS software package, version 22.0, for Windows (SPSS Inc., Armonk, USA). A general linear model was used to compare the means of the lung functional indices and PSQI scores between the group of patients after recovery from mild-to-moderate COVID-19 and the control group, after adjusting for subjects’ age, body mass index (BMI), sex, smoking, and alcohol consumption. In addition, to find the relationship between sleep quality and pulmonary function, the participants were categorized into three groups based on sleep duration and efficiency (<6, 6–8, and >8 h and >85%, 75%–85%, and <75%, respectively). The pulmonary functional indices associated with COVID-19, including FVC (%pred), FEV1 (%pred), FEV3 (%pred), FEF 200–1200 (L/s), FEF 200–1200 (%pred), PEF (L/s), and PEF (%pred), were then compared among the different groups of sleep duration or sleep efficiency using a general linear model. At that time, the analysis was adjusted for participant age, BMI, sex, smoking, alcohol consumption, and group (the control group or the group of the case recovering from COVID-19). The threshold of statistical significance was p<0.05.
RESULTS
Characteristics of the participants
Table 1 presents the characteristics of the participants, including age, sex, medical report, number of COVID-19 vaccine doses administered, SpO2 levels during infection, body size indices, smoking, alcohol consumption, and days from the day of the negative COVID-19 test to the examination day.
Table 1
Characteristics of the participants Statistical analysis
| Characteristics | All (N=160) | Cases after recovery from COVID-19 (N=110) | Control (N=50) |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Age (years), mean (SD) | 23.3 (4.5) | 23.1 (4.3) | 23.6 (5.0) |
| Gender (male) | 139 (86.9) | 94 (85.5) | 45 (90.0) |
| Vaccination against COVID-19 (doses) | |||
| 1 | - | - | - |
| 2 | 20 (12.5) | 14 (12.7) | 6 (12.0) |
| 3 | 140 (87.5) | 96 (87.3) | 44 (88.0) |
| Medical history | |||
| Obstructive lung disease | - | - | - |
| Restrictive lung disease | - | - | - |
| Other disease | - | - | - |
| Oxygen saturation levels, mean (SD) | 97.0 (2.0) | 96.6 (2.1) | 98.0 (1.0)* |
| Height (cm), mean (SD) | 168.8 (5.8) | 169.1 (5.5) | 168.1 (6.4) |
| Weight (kg), mean (SD) | 63.9 (8.2) | 63.8 (8.1) | 63.9 (8.3) |
| BMI (kg/m2), mean (SD) | 22.4 (2.3) | 22.3 (2.3) | 22.6 (2.3) |
| Smoking | 41 (25.6) | 27 (24.5) | 14 (28.0) |
| Alcohol consumption | 136 (85.0) | 93 (84.5) | 43 (86.0) |
| Days from the date of negative test for COVID-19 to the examination day, mean (SD) | 48.4 (25.6) |
The mean age of the participants was 23.3 years, and all participants had received at least two doses of the COVID-19 vaccine. The mean of SpO2 value was 97.0%, and no participant had a medical history of obstructive and restrictive lung diseases or other diseases. Among them, 86.9% were men, with 41 participants who smoked, constituting 25.6% of the total, while 85.0% of the participants reported alcohol consumption. The mean of BMI value was within the normal range (<25 kg/m2). The mean duration from the negative COVID-19 test results to the examination day was 48.4 days, with a range of 9 to 93 days. There were no significant differences in any characteristics between the groups that had recovered from COVID-19 and the control group, except for the SpO2 level (p<0.05) (Table 1).
Comparisons of pulmonary functional indices between the group of patients after recovery from COVID-19 and the control group
Table 2 shows the results of comparisons of the adjusted means of the pulmonary functional indices between the group of patients after recovery from COVID-19 and the control group. The adjusted means of FVC (%pred), FEV1 (%pred), FEV3 (%pred), FEF 200–1200 (L/s), FEF 200–1200 (%pred), PEF (L/s), and PEF (%pred) were significantly lower in the group of patients who had recovered from mild-to-moderate COVID-19 compared to the control group. However, there was no significant difference between the groups in terms of FEV1/FVC, FVC (L), and FEV1 (L/s) after recovery from mild-to-moderate COVID-19 compared to the control group for FEV1/FVC, FVC (L), and FEV1 (L/s) (Table 2).
Table 2
Comparisons of pulmonary functional indices between the group of patients after recovery from mild-to-moderate COVID-19 and the control group
| Pulmonary functional indices | Control (N=50) | Cases after recovery from COVID-19 (N=110) | p* | ||
|---|---|---|---|---|---|
| Adjusted Mean | SE | Adjusted Mean | SE | ||
| FVC (L) | 4.0 | 0.1 | 4.0 | 0.0 | 0.272 |
| FVC (%pred) | 90.3 | 1.6 | 86.2 | 1.1 | 0.031 |
| FEV1 (L/s) | 3.5 | 0.1 | 3.5 | 0.0 | 0.298 |
| FEV1 (%pred) | 93.0 | 1.5 | 87.8 | 1.0 | 0.006 |
| FEV1/FVC (%) | 87.8 | 1.0 | 87.9 | 0.6 | 0.903 |
| FEF 25–75% (L/s) | 4.3 | 0.1 | 4.1 | 0.1 | 0.405 |
| FEF 25–75% (%pred) | 97.8 | 2.9 | 92.2 | 1.9 | 0.106 |
| FEV3 (L/s) | 4.0 | 0.1 | 3.9 | 0.1 | 0.610 |
| FEV3 (%pred) | 89.7 | 1.4 | 84.9 | 1.0 | 0.006 |
| FEF 200–1200 (L/s) | 6.7 | 0.2 | 5.8 | 0.1 | 0.010 |
| FEF 200–1200 (%pred) | 85.9 | 2.7 | 72.6 | 1.8 | 0.0001 |
| PEF (L/s) | 7.4 | 0.2 | 6.6 | 0.1 | 0.0003 |
| PEF (%pred) | 88.8 | 2.3 | 76.5 | 1.5 | 0.00001 |
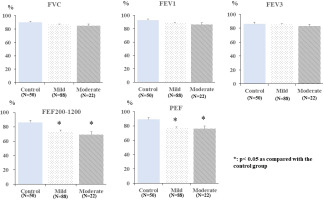
To assess the relationships between mild-to-moderate COVID-19 and lung function, a general linear model was used to compare the adjusted means of FVC (%pred), FEV1 (%pred), FEV3 (%pred), FEF 200–1200 (%pred), and PEF (%pred) among the groups of patients after recovery from mild-to-moderate COVID-19 and the control group. The analysis indicated no significant difference in the adjusted means of FVC (%pred), FEV1 (%pred), and FEV3 (%pred) among the three groups of mild-to-moderate COVID-19, and control groups (p>0.05).
However, the adjusted means of PEF (%pred) and FEF 200–1200 (%pred) were found to be significantly lower in the group of patients after recovery from mild-to-moderate COVID-19 when compared with the control group (p<0.05). No significant difference was observed in the adjusted mean of PEF (%pred) and FEF 200–1200 (%pred) between the groups after recovery from mild-to- moderate COVID-19 (Figure 1).
Comparisons of PSQI scores between the patients after recovery from COVID-19 and the control group
Table 3 presents the results of comparisons of the adjusted mean scores for subjective sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbances, use of sleep medication, daytime dysfunction, and overall PSQI scores between the groups of patients after recovery from COVID-19 and the control group, using a general linear model.
Table 3
Comparisons of PSQI scores between the control group and the group of patients after recovery from mild-to-moderate COVID-19
| PSQI scores | Control (N=50) | Cases after COVID-19 infection (N=110) | p* | ||
|---|---|---|---|---|---|
| Adjusted Mean | SE | Adjusted Mean | SE | ||
| Subjective sleep quality | 0.348 | 0.109 | 0.524 | 0.073 | 0.186 |
| Sleep latency | 0.823 | 0.115 | 0.790 | 0.077 | 0.813 |
| Sleep duration | 1.507 | 0.104 | 1.979 | 0.070 | 0.0001 |
| Sleep efficiency | 0.119 | 0.076 | 0.310 | 0.051 | 0.041 |
| Sleep disturbance | 0.360 | 0.068 | 0.345 | 0.046 | 0.857 |
| Use of sleep medication | 1.487 | 0.166 | 1.161 | 0.111 | 0.105 |
| Daytime dysfunction | 0.712 | 0.111 | 0.567 | 0.075 | 0.286 |
| Overall score | 5.355 | 0.327 | 5.675 | 0.220 | 0.420 |
The adjusted mean scores for sleep duration and sleep efficiency were significantly higher in the group of patients after recovery from COVID-19 compared to the control group (p<0.05). However, there was no significant difference in the scores for other subscales and overall PSQI scores between the group of patients after recovery from COVID-19 and the control group (p>0.05) (Table 3).
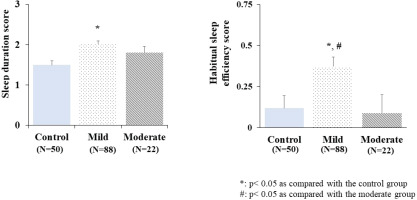
Additionally, to explore the relationship between mild-to-moderate COVID-19 and sleep quality, sleep duration, and sleep efficiency scores were compared among the groups of patients recovering from mild-to-moderate COVID-19 and the control group. The results are presented in Figure 2. The adjusted mean sleep duration score was significantly higher in the group of patients recovering from mild COVID-19 than in the control group (p<0.05). Similarly, the group of patients after recovery from mild COVID-19 showed a significant increase in the adjusted mean of sleep efficiency score when compared with the group of patients after recovery from moderate COVID-19 and the control group (p<0.05). There was no significant difference in either the adjusted mean sleep duration score or sleep efficiency score between the group of patients after recovery from moderate COVID-19 and the control group (p>0.05) (Figure 2). These results indicated that the impacts of COVID-19 on sleep duration and sleep efficiency were more prominent in the group of patients recovering from mild disease.
Relationships between the pulmonary functional index and sleep quality
The adjusted means of the pulmonary functional indices associated with COVID-19, including FVC (%pred), FEV1 (%pred), FEV3 (%pred), FEF 200–1200 (L/s), FEF 200–1200 (%pred), PEF (L/s), and PEF (%pred) were compared among the different groups of sleep duration or sleep efficiency using a general linear model and the results are presented in Table 4.
Table 4
Relationships between pulmonary functional indices and sleep duration and efficiency
| Pulmonary functional indices | Sleep duration | p | |||||
|---|---|---|---|---|---|---|---|
| <6 hours (N=64) | 6–8 hours (N=79) | >8 hours (N=17) | |||||
| Adjusted Mean | SE | Adjusted Mean | SE | Adjusted Mean | SE | ||
| FVC (%pred) | 87.1 | 1.4 | 89.2 | 1.2 | 80.5 | 2.8 | **,‡ |
| FEV1 (%pred) | 90.3 | 1.3 | 90.6 | 1.2 | 80.8 | 2.7 | *,‡‡ |
| FEV3 (%pred) | 86.5 | 1.3 | 87.7 | 1.1 | 80.1 | 2.6 | **,‡ |
| FEF 200–1200 (L/s) | 6.1 | 0.2 | 5.9 | 0.2 | 6.3 | 0.4 | |
| FEF 200–1200 (%pred) | 77.5 | 2.4 | 75.9 | 2.2 | 78.2 | 4.9 | |
| PEF (L/s) | 7.0 | 0.2 | 6.7 | 0.2 | 6.9 | 0.4 | |
| PEF (%pred) | 81.7 | 2.0 | 79.5 | 1.8 | 79.2 | 4.2 | |
| Sleep efficiency | |||||||
| <75% (N=7) | 75–85 % (N=25) | >85 % (N=128) | |||||
| FVC (%pred) | 89.0 | 4.4 | 87.9 | 2.2 | 87.3 | 1.0 | |
| FEV1 (%pred) | 94.9 | 4.3 | 90.8 | 2.2 | 88.9 | 0.9 | |
| FEV3 (%pred) | 88.1 | 4.0 | 86.9 | 2.0 | 86.2 | 0.9 | |
| FEF 200–1200 (L/s) | 6.9 | 0.6 | 5.9 | 0.3 | 6.1 | 0.1 | |
| FEF 200–1200 (%pred) | 91.0 | 7.4 | 75.0 | 3.7 | 76.4 | 1.6 | |
| PEF (L/s) | 7.6 | 0.6 | 6.7 | 0.3 | 6.8 | 0.1 | |
| PEF (%pred) | 91.2 | 6.3 | 80.2 | 3.2 | 79.8 | 1.4 | |
SE: standard error. Covariates for age, gender, BMI, smoking, alcohol drinking, COVID-19 infection status (yes/no).
* p<0.05 when compared between the group with sleep duration >8 hour and the group of sleep duration 6–8 hours.
** p<0.01 when compared between the group with sleep duration >8 hour and the group with sleep duration 6–8 hours.
The adjusted means of FVC (%pred), FEV1 (%pred), and FEV3 (%pred) in the group with sleep duration of <6 h and >8 h were lower than those in the group with sleep duration 6–8 h. However, a significant decrease was observed only in the group with sleep duration >8 h (p<0.05). There was no significant difference in the adjusted means of FE 200–1200 (L/s), FEF 200–1200 (%pred), PEF (L/s), and PEF (%pred) among different groups of sleep duration (p>0.05). Furthermore, no significant difference was found in any pulmonary functional indices, including FVC (%pred), FEV1 (%pred), FEV3 (%pred), FEF 200–1200 (L/s), FEF 200–1200 (%pred), PEF (L/s), and PEF (%pred), among the different groups of sleep efficiency (p>0.05) (Table 4).
DISCUSSION
Alterations of pulmonary function in the cases after recovery from COVID-19
In this study, decreased FVC (%pred), FEV1 (%pred), FEV3 (%pred), FEF 200–1200 (L/s), FEF 200–1200 (%pred), PEF (L/s), and PEF (%pred) were found in the group of patients after recovery from mild-to-moderate COVID-19 compared with the control group. This finding suggests that alterations in lung function are indicated by restrictive ventilatory dysfunction after recovery from mild-to-moderate COVID-19. Moreover, it was reported that FEF 200–1200, which has been considered a substitute for PEF and contributed to decreased PEF values, might reflect the late diagnosis of obstructive lung function in practice rather than the detection of early disease24. Taken together with our results, these data provide evidence of the impact of mild-to-moderate COVID-19 on lung function in the first three months after infection in young and middle-aged adults.
In a previous study, impairment of DLCO (%pred), total lung volume (TLC) (%pred), and FEF 50%–75% (%pred) were observed at 2 to 6 weeks after the onset of COVID-19 infection in patients with mean age of 49.1 ± 14.0 years. They also reported that this alteration was associated with the severity of the disease, with a significant decrease in DLCO and TLC values in the severe pneumonia group compared with those in the mild-to-moderate illness groups4. Huang et al.25 investigated the influence of COVID-19 on lung function in the early convalescence phase in patients aged 19–70 years and reported that reduced DLCO and TLC were found in more than half of the COVID-19 patients at one month after discharge from the hospital, with a higher incidence of DLCO impairment and a decrease in the severe group; however, the control group was not included in these studies3,4,25. In a study by You et al.3, a restrictive ventilatory defect indicated by decreased FVC (%pred) was found in nearly half of patients aged 28–67 years at 5 to 9 weeks after recovery from COVID-19 infection, but these alterations were not associated with the severity of the disease. In a Swedish study, Mogensen et al.9 reported no evidence of mild-to-moderate COVID-19 affecting lung function, as indicated by FVC, FEV1, and FEV1/FVC in young adults. In the present study, no patients aged >40 years or with a medical history were collected, and impaired pulmonary function was observed in the group of patients after recovery from mild-to-moderate COVID-19. To the best of our knowledge, this is the first study on the impact of COVID-19 on pulmonary function in healthy young and middle-aged adults, in Vietnam.
Additionally, we observed no significant differences in pulmonary function indices between the two groups after recovery from mild-to-moderate COVID-19. To explain the impairment of lung function that did not correlate with the severity of illness or residual imaging changes, Huang et al.25 hypothesized that the use of corticosteroids may improve the prognosis of patients with COVID-19. However, we did not find any significant differences in pulmonary function indices between the two groups of patients who used corticosteroids and those who did not (unpublished data). Furthermore, reduced lung function is primarily observed in patients with severe COVID-194,25. Although a decline in lung function was noted after COVID-19 infection, patients with severe COVID-19 were not included in this study. Therefore, we planned to additionally recruit adults after recovery from the severe COVID-19 to determine whether association between changes in pulmonary function and the disease severity in young and middle-aged adults.
Alterations of sleep quality and its associations with pulmonary function in the cases after recovery from COVID-19
In this study, we also observed an increase in sleep duration and a decrease in sleep efficiency in the group of patients after recovery from COVID-19, particularly in those who had experienced mild disease. In a previous study evaluating sleep quality during the first six months after recovery from COVID-19 infection, Kalamara et al.26 reported persistent poor sleep quality at 1, 3, and 6 months. In a study in Romania, Munteanu et al.11 found an increased incidence of sleep quality problems at 6 months after recovery from COVID-19, with a higher rate in the mild-to-moderate groups. Jackson et al.12 reported that poor sleep quality persisted in cases after 2 to 14 months of recovery from COVID-19. Although sleep disorders are frequently observed in older patients27, sleep disorders are more prominent in younger individuals who recovered from COVID-19 infection26-28. Therefore, further study is required to evaluate the consistent alteration of sleep quality in the present young and middle-aged adults who recovered from mild-to-moderate COVID-19.
Moreover, decreased FVC (%pred), FEV1 (%pred), and FEV3 (%pred) were observed in the group of patients who slept >8 h per day in this study. In a previous study, Jackson et al.12 reported lower FEV1 (%pred) and FVC (%pred) in participants with a deterioration in their sleep quality indicated by an overall PSQI score ≥5. However, they did not show relationships between the components of PSQI, including sleep duration, sleep efficiency, and lung function, in patients after recovery from COVID-19. In addition, relationships between FVC (%pred) and FEV1 (%pred) and COVID-19 were not mentioned in the study12. The adjusted means of FVC (%pred) and FEV1 (%pred) were compared between the group of cases with higher and lower overall PSQI scores (PSQI ≥5 and <5), but no significant difference was observed in the present study. Because our study was focused on young and middle-aged adults who recovered from COVID-19 for the first 3 months, it is different from that of Jackson et al.12. They reported the alteration of pulmonary function and sleep quality in patients with a mean age of 58 years (SD=13) and recovered from COVID-19 for 2 to 14 months. These factors might partly contribute to the different results between our study and the previous study. Taken together, our findings provide additional evidence regarding the clinical associations of reduced lung functions and poor sleep quality, particularly sleep duration, among patients recovering from COVID-19. In addition, pulmonary rehabilitation has been reported to improve sleep quality, mental and physical health in patients after recovery from COVID-1929. A pulmonary rehabilitation program should be conducted for the patients in the present study, particularly the patients who showed reduced lung function and poor sleep quality.
Possible mechanism of COVID-19 impacts on pulmonary function and its association with sleep disorders
The lung is likely the primary target organ for COVID-19, exhibiting various pathologies, including lung epithelial and endothelial injury, leading to edema, desquamation of alveolar epithelial cells, hyaline membrane formation, inflammation, hemorrhage, capillary damage, collapse of alveoli, alveolar septal fibrous proliferation, and pulmonary consolidation30-32. These factors may contribute to the impairment of lung function in patients with COVID-19. Additionally, COVID-19 induced alterations in sleep patterns may impact the immune response profile, leading to changes in immune cell number or distribution of immune cell phenotypes, decreased immunization efficacy33, or systemic inflammation34. These factors can exacerbate lung inflammation during COVID-19 infection.
Moreover, COVID-19 can involve multiple organs and affect many systems, indicating symptoms not only related to respiratory or sleep quality but also other aspects, including physical, cognitive, and mental health after hospitalization34, which have been reported to be associated with pulmonary function or sleep quality12,35. Therefore, network structures of pulmonary function and associated factors such as sleep quality and mental health, should be analyzed in the future for COVID-19 patients in Vietnam.
Limitations
The findings of this study should be interpreted in the context of certain limitations. This study has a small sample size. Another limitation in the present study should be mentioned that the participants were collected in a single center in Vietnam. Moreover, we used PSQI to evaluate sleep quality of the participants, which can include subjectivity. In addition, we adjusted five factors including subjects’ age, BMI, sex, smoking, and alcohol consumption to eliminate confounding between COVID-19 infection and pulmonary function, and sleep quality. Pre-existing health conditions have been reported that associated with pulmonary function and sleep quality in a previous study12. We also collected information on pre-existing health conditions and confirmed that there was no significant difference for the scores of the spirometry test or PSQI between the group with and without pre-existing health conditions in the present study. However, there are still other factors which may influence to pulmonary function and sleep quality such as quality of life, and physical activity36. Therefore, a larger study should be conducted using objectives methods such as actigraphy or polysomnography, with an emphasis on factors influencing pulmonary function and sleep quality.
CONCLUSIONS
Alterations of pulmonary function and sleep quality were observed in young to middle-aged adults for the first 3 months after recovery from mild-to-moderate COVID-19. In addition, reduced pulmonary function might partly be associated with poor sleep quality, particularly sleep duration among patients recovering from COVID-19. Pulmonary rehabilitation program and a follow-up study on pulmonary function and sleep quality for these subjects would be necessary in the future.