INTRODUCTION
Anthracosis belongs to the greater group of environmental lung diseases, called pneumoconiosis1. Although quite frequent in Asia2,3, only few cases are reported in Greece annually. We present a case study in order to highlight the diagnostic challenges and clinical complications that we encountered.
CASE PRESENTATION
A 71-year-old refugee from Afghanistan presented with acute hypoxemic respiratory failure. She had recently been hospitalized due to fever, dyspnea and intense wheezing, that were treated with intravenous methylprednisolone. She was not a smoker; however, she was exposed to biomass fuels in a poorly ventilated household for at least 50 years and reported having chronic cough and dyspnea the last 5 years. She was also hospitalized 3 years ago for a lower respiratory tract infection.
Her vital signs included a pulse rate of 84 beats per minute, body temperature of 37℃, and respiratory rate of 22 breaths per minute. Blood saturation was approximately 90% with a nasal cannula oxygen supply of 4 L/min, while blood pressure was normal. Physical examination of the chest revealed wheezing sounds during respiration in both lungs.
Complete blood count showed elevated leukocytes (19400/μL) and neutrophils (93.8%), possibly due to recent corticosteroid treatment. C-reactive protein was slightly elevated at 4.03 mg/dL (normal range <0.8 mg/dL). B-natriuretic peptide, angiotensin converting enzyme and procalcitonin values were normal. Auto-antibodies screen was also negative.
Pulmonary function tests showed restrictive ventilatory defects combined with small-airway obstruction with forced vital capacity (FVC) of 1.17 L (50% of predicted value), forced expiratory volume in 1 second (FEV1) of 0.85 L (44% of predicted value), FEV1/FVC ratio of 75.5%, and forced expiratory flow between 25% and 75% of vital capacity (FEF25–75%) of 0.76 L (30% of predicted values).
Chest X-ray revealed masses in both hilar areas. Chest computed tomography (CT) (Figure 1) revealed:
Figure 1
Chest computed tomography scan (CT) (A) – (D). These images reveal several enlarged mediastinal lymph nodes (1), bilateral lung nodules (2), ground glass infiltration (3), mosaic attenuation pattern (4), and bronchiectasis in the apical part of the left upper lobe (LUL) with fibrotic features and attraction of the mediastinal pleura (5), stenosis of RUL bronchus (6)
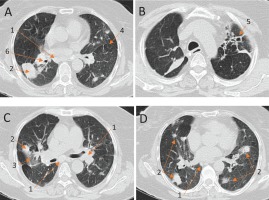
Multiple enlarged mediastinal lymph nodes; many of them presented peripheral calcification.
A large disparate, calcified consolidation in the posterior part of the right upper lobe (RUL), which attracted the side pleura and caused stenosis of the bronchus for the right upper and the middle lobe.
Bilateral lung nodules of various sizes, with the largest presenting dimensions of 4×2.4 cm.
Ground glass infiltration in the RUL.
Bronchiectasis in the apical part of the left upper lobe (LUL) with fibrotic features and attraction of the mediastinal pleura.
Mosaic attenuation pattern.
Τhe possibility of malignancy was thoroughly investigated. Sputum cytology was negative for neoplastic cells. Bronchoscopy was performed revealing highly inflammatory and hemorrhagic mucous membrane and mild stenosis of the RUL bronchus. Bronchial biopsy was taken from the stenotic region of the RUL and revealed inflammation of the mucosa and hyperplastic lesions, without evidence of dysplasia. Cytology revealed abundance of inflammatory cells, mainly alveolar macrophages, as well as squamous and bronchial epithelium cells. Bronchoalveolar lavage (BAL) cultures for common bacteria, Mycobacterium tuberculosis, Histoplasma capsulatum, Nocardia and Pneumocystis jiroveci were negative. Serum galactomannan values were also negative.
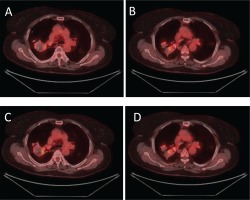
The next step of the diagnostic approach was to order a positron emission tomography scan CT (PET/CT) that revealed bilateral, hypermetabolic, nodulus lesions. The largest nodule was sited in the RUL and presented an intake of 4.8 maximum standard unit value (SUVMAX), making the diagnosis of an inflammatory, rather than a malignant disease, more likely (Figure 2).
Figure 2
PET computed tomography scan (PET/CT) (A) – (D). These images reveal bilateral, hypermetabolic, nodulus lesions, indicative of inflammatory/infectious disease
CT guided fine needle aspiration (FNA) from the largest hypermetabolic nodule was then performed. The FNA cytologic examination was conclusive, as macrophage cells containing particles of soot were evident.
Subsequently, during her hospitalization, the patient developed tachycardia (about 120 bpm), accompanied with elevated oxygen needs, pain on lower limp palpation and T-wave inversion in the V1, V2, V3 chest leads. The patient’s Well’s and Genova scores were 6 and 10 points respectively, determining a moderate risk for PE. D-dimmers levels were also elevated and a CT pulmonary angiography (CTPA) was performed with the upcoming findings (Figure 3):
Figure 3
CT pulmonary angiography (CTPA) (A) – (D) These images reveal filling defects in multiple vessels of each lobe in both lungs.
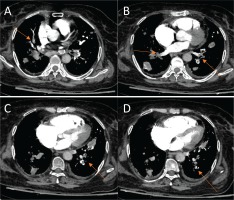
Filling defects in multiple vessels of the LUL and the left lower lobe (LLL).
Complete defect of perfusion for multiple vessels of the posterior part of the RUL, the middle lobe (ML) and the right lower lobe (RLL).
The patient was already on prophylaxis with enoxaparin, as she was bedridden, with oxygen needs, throughout a long hospitalization and subsequently begun treatment with therapeutic dose of enoxaparin for 5 days, that was switched to rivaroxaban, as clinical stability was ensured.
Pro-coagulant factors, other than immobilization during her hospitalization, were not detected. Protein C, protein S, antithrombin III and homocysteine levels were normal, and she was negative for Factor V Leiden mutation.
The patient was treated with inhaled corticosteroid plus long-acting beta-agonist (ICS/LABA) and oral methylprednisolone, given the fact that severe wheezing episodes were very frequent. Instructions for gradual withdrawal of corticosteroids were given to the patient.
DISCUSSION
Anthracosis seems to occur following accumulation of carbon in the lungs due to exposure to coal dust particles4. It may also be complicated with mucosal proliferation, luminal obliteration and/or obstruction, resulting in the condition called anthracofibrosis5.
Small size inhaled particles (0.5–5 μm), which are either engulfed by macrophages in the submucosa or remain in the bronchial tree due to deficient mucociliary clearance, lead to bronchial epithelial cells deposition6. Black macules are formed that gradually progress to the nodules of the surrounding lung parenchyma, causing progressive massive fibrosis (PMF)7, thus leading to bronchial wall hypertrophy, lumina narrowing and pulmonary dysfunction6. Coexistence of Tuberculosis (TB) and athracofibrosis might also be relevant as lymph nodes accumulated by carbon and silica might rupture into the adjoining tracheobronchial tree as soon as they get infected by M. tuberculosis, leading to black pigmentation and subsequent inflammation8.
Given the pathogenic importance of coal exposure, anthracosis appears with increasing incidence in industrialized countries, especially in Asia, with most cases of anthracofibrosis induced by indoor pollution recorded in Korea6 and Iran2. In Europe, anthracosis was common in coal workers between the 1960s and 1980s, but recent reports show that it is currently prevalent in farmers and rural dwellers9. Due to low physical strength, and the progressive nature of the disease, older individuals seem to be more vulnerable10. Meanwhile, a female preponderance has been reported by most researchers2.
Diagnosis of anthracofibrosis is a complex procedure as it is a rare condition with limited literature in Europe. Clinical symptoms are barely specific. Most patients present with dyspnea (90–100% of cases), cough (29.8–83.6% of cases)2 and wheezing6. Hemoptysis or non-specific chest pain, constitutional symptoms, and sputum production, both black and watery, are also occasionally observed. There are also reports of new onset weight loss or fever, enlarged mediastinal lymph nodes, and subsequent complications such as vocal cord paralysis or broncholithiasis as initial symptoms6. Normal physical examination is also noted in some cases6.
Τhe usual chest CT findings, according to Kahkousee et al.1, include enlarged lymph nodes, hyperattenuation of the lung parenchyma, and multisegmental atelectasis. Bronchial narrowing, peribronchial cuffing, consolidation, mosaic lung attenuation and pulmonary nodules are also common1.
Moreover, bronchoscopy is considered to be the gold standard diagnostic tool, with multiple pigmented anthracotic lesions and bronchial stenosis as principal findings6.
Hοwever, anthracosis is not always localized in bronchi and may spread to the parenchyma1. Histopathological findings in biopsy samples of anthracofibrosis patients revealed infiltration of non-specific mononuclear inflammatory cells with typical intracellular and extracellular black particles observed in the epithelium and stroma6.
In our case, the cytological finding of macrophages containing soot particles, along with a suitable radiologic image was sufficient to set the final diagnosis, since bronchoscopy did not reveal the typical findings and other possible diagnoses were excluded.
Αnthracofibrosis is known to be associated with a number of pulmonary diseases. Among them, tuberculosis (TB) seems to be the most dominant8 in 15.8% of the anthracotic patients11. Chronic obstructive pulmonary disease (COPD)6, respiratory infections12 and sarcoidosis13 have also been reported as related conditions, while the association between anthracofibrosis and asthma is under investigation2.
Pulmonary function tests14 show controversial findings with approximately two-thirds of patients manifesting obstruction, and one-third restriction6. Specific association between lung cancer and anthracofibrosis has not been found yet, although adenocarcinoma seems to be the most prevalent histopathological type in patients with antracosis15.
Moreover, pulmonary embolism is a condition which seems to be prevalent in chronic respiratory disease (CRD) patients3. Antracofibrosis seems to be the second most common (25%) chronic lung disease among patients with pulmonary embolism3 after COPD (32%), according to a recent study in Korea. Immobility, chronic inflammation, recurrent infections and other comorbidities seem to create pro-coagulant conditions, responsible for this clinical complication.
In particular, anthracofibrotic lungs contain granulation tissue and fibrotic lesions6, indicative of hyperactivity of the tissue factor which is a key element in the coagulation pathway16. In addition, squamous metaplasia, dysplasia and hyper vascularization have also been occasionally reported6, pointing to a pre-neoplastic condition which is also characterized by hypercoagulant activity. Moreover, there have been reports of elevated factor VIII levels, associated with endothelial injury in respiratory failure patients17, while BAL fluids of fibrotic patients have shown increased levels of plasminogen activator inhibitor18. Ιncreased platelet activation, mediating inflammatory and thrombotic responses, have also been demonstrated in a number of respiratory diseases19. The aforementioned factors introduce a hypercoagulant activity in any severe lung disease, including anthracofibrosis.
Lastly, specific treatment for anthracofibrosis has not been established yet. The symptomatic relief includes antibiotics for concomitant respiratory infections, bronchodilators, mucolytic agents and inhaled corticosteroids6. Antituberculosis treatment in TB endemic regions and combination of corticosteroids with tamoxifen as anti-fibrotic agents have shown promising results, while endobronchial stent placement, for patients with severe bronchial stenosis is also under investigation6. Anticoagulants in prophylaxis dose are occasionally administrated in hospitalized patients, due to their deteriorated general condition and their increased oxygen needs, which keep them bedridden and physically restricted. However, in the context of outpatient thrombosis prevention, preemptive initiation of anticoagulants remains controversial.
CONCLUSION
Extensive imaging and laboratory examinations might be required to establish the diagnosis of anthracofibrosis. Although bronchoscopy is the gold standard, in our case it was non diagnostic and it was the cytologic examination of the aspirate that set the diagnosis. In this direction, anthracosis should always be considered as a potential diagnosis in patients with coal exposure history, particularly when other profound causes are excluded and the differential diagnosis remains confusing. In the meantime, the risk of pulmonary embolism remains increased in the context of chronic inflammation and should always be considered as a possible complication.


